and Alessandra Cancellieri2
(1)
Department of Radiology, Bellaria Hospital, Bologna, Italy
(2)
Department of Pathology, Maggiore Hospital, Bologna, Italy
Case-Based Glossary with Tips and Tricks
(3)
Department of Radiology, Bellaria Hospital, Bologna, Italy
Clinical features | Marco Patelli | |
Radiology | Giorgia Dalpiaz Marta Fiscaletti Marco Piolanti | |
Pathology | Alessandra Cancellieri |
Air crescent sign | Page 252 | |
Air trapping | Page 255 | |
Angiogram sign | Page 257 | |
Beaded septum sign | Page 259 | |
Bubble-like lucencies | Page 261 | |
Butterfly sign | Page 263 | |
Cheerio sign | Page 265 | |
Comet tail sign | Page 267 | |
Crazy paving | Page 269 | |
Finger-in-glove sign | Page 271 | |
Galaxy sign | Page 273 | |
Halo sign | Page 275 | |
Head-cheese sign | Page 277 | |
Reversed batwing sign | Page 279 | |
Reversed halo sign | Page 281 | |
Snowflake sign | Page 283 | |
Tree in bud, bronchiolar | Page 285 | |
Tree in bud, vascular | Page 287 |
Air Crescent Sign
Meniscus or cap sign

Clinical History
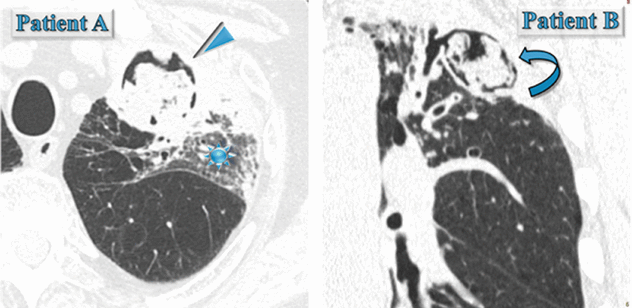
(Patient A) Male in his 70s with fever, cough, and hemoptysis: immunocompromised patient with neutropenia.
(Patient B) Male in his 70s, he underwent coronary artery bypass due to ischemic heart disease. No dyspnea, immunocompetent, BPCO, and persistent productive cough.
HRCT
(Patient A) In the left upper lobe, axial HRCT image shows rounded area of consolidation surrounded by a very thin black area (air crescent sign) (►). An area of ground-glass opacity (GGO) also coexists in the same lobe ( ).
).
 ).
).(Patient B) In the left upper lobe, coronal HRCT image shows cystic bronchiectases with intracavitary material surrounded by a crescent-shaped thin black area (air crescent sign) (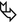 ).
).
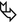 ).
).
Causes of Air Crescent Sign
Common
Angioinvasive aspergillosis
Mycetoma (also defined aspergilloma)
Rare
Abscess
Cavitary neoplasm
Granulomatosis with polyangiitis (Wegener granulomatosis)
Hematoma
Hydatid cyst
Pneumocystis jiroveci pneumonia
Tuberculosis (TB)
Tips and Tricks
Please pay attention to the state of the patient immunocompetence and if there was a preexisting cystic or cavitary lung disease in a previous CT. As a matter of fact, invasive aspergillosis should be suspected in any patient with neutropenia who develops a fever and presents the air crescent sign inside a consolidation or mass (Patient A). On the contrary, the air crescent sign of mycetoma, also referred to as the Monad sign, is seen in an immunocompetent host with preexisting cystic or cavitary lung disease, usually from tuberculosis or sarcoidosis (Patient B).
Mycetoma is usually located in the upper lobes. Lower lobes and multifocal distribution should increase the suspicion of a different diagnosis.
Management and Diagnosis
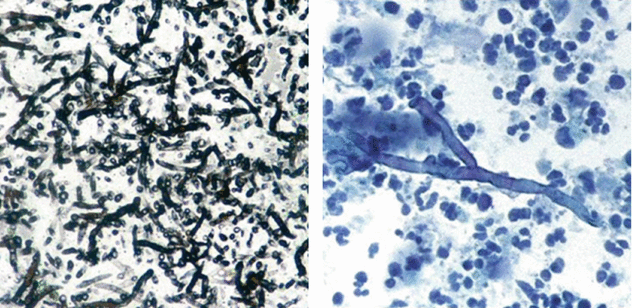
Both patients were positive for serum biomarker galactomannan together with the presence of Aspergillus in the bronchoalveolar lavage (BAL) (septate hyphae branching at 45° on a necrotic background; see the images below).
Final diagnosis of Patient A: angioinvasive aspergillosis
Final diagnosis of Patient B: mycetoma

Pearls
Air crescent sign is recognized as a crescent-shaped or circumferential area of radiolucency within a parenchymal consolidation or nodular opacity. This sign is often seen in two types of Aspergillus infection: angioinvasive and mycetoma.
Pathogenesis of air crescent sign in angioinvasive Aspergillus infection. It is caused by parenchymal cavitation, which typically occurs 2 weeks after the detection of the initial radiographic abnormality. The nodules are composed of infected hemorrhagic and infarcted lung tissue. As the neutrophil count recovers and the patient mounts an immune response, peripheral reabsorption of necrotic tissue causes the retraction of the infarcted center, and air fills the space in between. This creates an air crescent within the nodules and is a good prognostic finding because it marks the recovery phase of the infection. This sign is seen in approximately 50 % of patients. Angioinvasive Aspergillus infection is often fatal.
Pathogenesis of air crescent sign in mycetoma (aspergilloma). It is caused by the presence of a fungus ball inside a cavity separated from the wall of the cavity by an airspace of variable size. Air crescent sign in mycetoma, also referred to as the Monad sign, was first described in 1954 by Pesle and Monod. It is seen in an immunocompetent host with preexisting cystic or cavitary lung disease, usually from tuberculosis or sarcoidosis. The radiographic appearance is often that of a gravity-dependent mass within a preexisting cavity.
Hansell DM (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697
Nitschke A (2013) Monod sign. J Thorac Imaging 28:W120
Franquet T (2001) Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics 21(4):825
Air Trapping
Gas trapping

Clinical History
Female in her 60s, ex-smoker. Bronchial asthma from 15 years, on continuous therapy during the last 3 years. Clinical reevaluation for worsening cough shows bronchial obstruction, not reversible.
HRCT
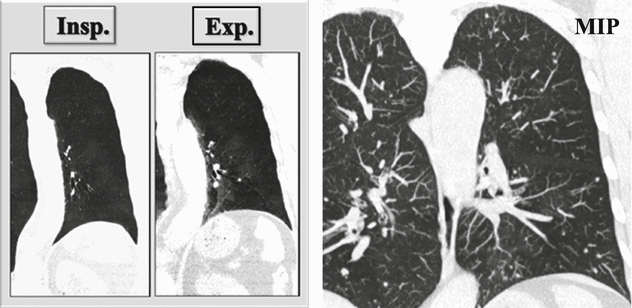
Coronal inspiratory (Insp.) and expiratory (Exp.) CT images with minimum intensity projection (MinIP) algorithm show patchy areas of black and white aspect due to air trapping. Coronal CT image with maximum intensity projection (MIP) shows small solid nodules with random distribution.

Causes of Air Trapping
With Bronchiectasis
Constrictive bronchiolitis (CB)
Congenital conditions (cystic fibrosis, primary ciliary dyskinesia, Swyer–James Syndrome – SJS,
Williams–Campbell syndrome)
Infection (atypical mycobacteria, tuberculosis, ABPA)
With Interstitial Lung Disease
Constrictive bronchiolitis (CB), DIPNECH, sarcoidosis, hypersensitivity pneumonitis (HP), andcollagen vascular disease (CVD)
Tips and Tricks
In our patient air trapping is not associated with bronchiectasis but with interstitial lung disease (nodules) displaying solid density and random distribution. The presence of random nodules rules out the diagnosis of sarcoidosis or hypersensitivity pneumonitis.
The coexistence of patchy areas of air trapping with solid random nodules supports the diagnosis of constrictive bronchiolitis in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH).
Management and Diagnosis
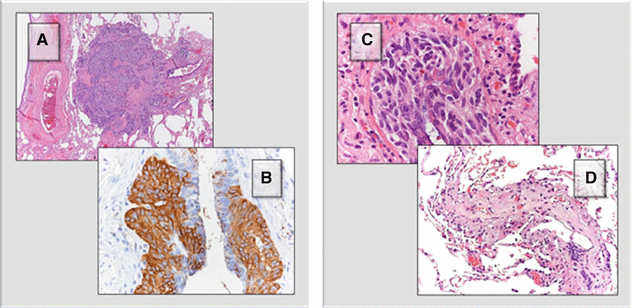
The patient underwent a surgical lung biopsy. Nodules (A) are composed of a proliferation of neuroendocrine cells, as demonstrated by the positivity with anti-chromogranin antiserum (B). The neuroendocrine proliferation can also consist in a linear growth within the airway wall (C). As a result, fibrosis of the bronchioles can ensue, both in the form of stenosis and complete obliteration of the lumen (D).
Final diagnosis: diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH)

Pearls
Air trapping is the retention of air in the lung distal to an obstruction (usually partial). Air trapping is seen on expiration CT scans as parenchymal areas with less than normal increase in attenuation and lack of volume reduction. Comparison between inspiratory and expiratory CT scans can be helpful when air trapping is subtle or diffuse.
Pathogenesis. Small-airway disease results from different causes (please see the table above).
DIPNECH, when associated with bronchiolar fibrosis, is also known as Aguayo–Miller syndrome after the name of the authors who first published a clinical series of six cases in 1992. The majority of patients presenting with DIPNECH are middle-aged females with symptoms of cough and dyspnea, obstructive abnormalities on pulmonary function testing, and radiographic imaging showing pulmonary nodules, air trapping, and mild bronchiectases. In general, the clinical course remains stable; however, progression to respiratory failure does occur. Long-term follow-up and treatment remain unclear. Transbronchial biopsy in search of a specific etiology is often a first step. If this remains negative, then a video-assisted thoracoscopic surgical biopsy may be necessary. Please also refer to DIPNECH in the Dark Lung Diseases chapter.
Hansell DM (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697
Kligerman SJ (2015) Mosaic attenuation: etiology, methods of differentiation, and pitfalls. Radiographics 35(5):1360
Nassar AA (2011) Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a systematic overview. Am J Respir Crit Care Med 184(1):8
Angiogram Sign
Lightning sign (n.d.e.)

Clinical History
Woman in her 70s, previous smoker with dry cough, asthenia, and chest right pain for 6 months. Recent diagnosis of autoimmune disease. The chest radiography shows consolidation not responsive to medical treatment (“non-resolving pneumonia”).
CT
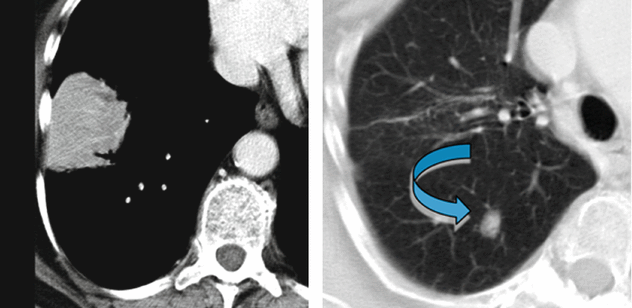
In the lower right lobe, CT shows consolidation with visibility of patent white vessels crossing the lesion (angiogram sign). In the upper right lobe, a parenchymal nodule is also visible (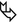 ).
).
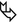 ).
).
Causes of Angiogram Sign
Common Disease
Adenocarcinoma
Pneumonia
Rare Disease
Lipoid pneumonia
Lymphoma
Metastasis of gastrointestinal adenocarcinomas
Obstructive pneumonitis due to central lung tumors
Tips and Tricks
Please note that the consolidation presents a mass-like aspect without bronchogram sign. The vessels inside the lesion (angiogram sign) appear thinned and stretched. This feature suggests the presence of material that occupies the alveoli with mass effect.
In our case the mass-like consolidation with angiogram sign is associated with a nodule. These imaging features are nonspecific and may resemble a variety of benign and malignant chest disorders including adenocarcinoma. However, the diagnosis of pulmonary MALT lymphoma should be considered in patient with the imaging features described above and with a history of autoimmune disorder.
Management and Diagnosis
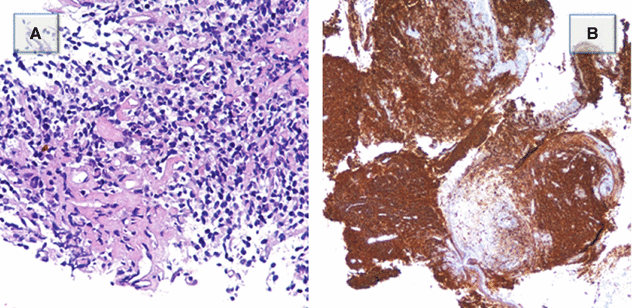
The bronchoscopy showed endobronchial vegetation into the proximal tract of the lower right bronchial pyramid. Pathologic analysis showed fragments of a diffuse proliferation composed of small centrocyte-like lymphocytes (Figure A). The proliferating lymphocytes are CD20 positive, a feature consistent with B-cell derivation (Figure B) (Courtesy of S. Damiani, Bellaria Hospital, University of Bologna).
Diagnosis: low-grade B-cell pulmonary lymphoma MALT

Pearls
Angiogram sign refers to the visualization of pulmonary vessels within an consolidation, on contrast-enhanced CT scanning. The vessels are prominently seen against a background of a relatively low-attenuation lesion. It has been initially described in 1990 by Im as a specific sign of lobar bronchoiolo-alveolar carcinoma. In the following years, this sign has been reported to be present in several different benign and malignant diseases (please see the table).
Pathogenesis. The vessels are prominently seen due to the variable low-attenuation density of the consolidation. CT low-attenuating lung consolidations with angiogram sign after intravenous contrast material administration may be due to the presence in the airspace of fat (lipoid pneumonia), mucus (obstructive pneumonia with abundant accumulation of secretions), necrosis (necrotizing pneumonia), or mucin (primary or metastatic mucinous adenocarcinoma) (please also refer to the Cheerio sign in this chapter).
Pulmonary MALToma does not present pathognomonic imaging features. The commonest radiological manifestations are pulmonary mass, or mass-like area of consolidation and multiple pulmonary nodules. Common associated features include air bronchograms, positive angiogram sign on contrast-enhanced CT, and halo of ground-glass opacity (halo sign). Please also refer to MALToma in the Alveolar Diseases chapter.
Im JG (1990) Lobar bronchioloalveolar carcinoma: “angiogram sign” on CT scans. Radiology 176(3):749
Beaded Septum Sign
Beaded appearance, nodular septal thickening

Clinical History
Man in his 20s with cough, dyspnea, and restrictive ventilatory defect at pulmonary function tests. The chest radiography shows multiple micronodules predominant in the upper and middle zone.
HRCT
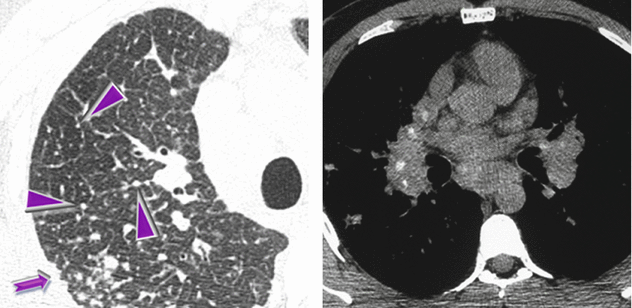
HRCT at the level of the right upper lobe shows diffuse nodular thickening of the interlobular septa (beaded septum sign ►) and subpleural micronodules (➨). Axial unenhanced CT (mediastinal window) shows bilateral hilar and subcarinal calcified lymph node enlargement.

Causes of Beaded Septum Sign
Common
Lymphangitic carcinomatosis (LC)
Rare
Amyloidosis
Lymphoproliferative disease (lymphoma, leukemia)
Sarcoidosis
Tips and Tricks
Small and dense nodules beaded some septa (see above, left) but also the subpleural space with the so-called perilymphatic distribution (“avid of pleura”). This pattern is most typical of sarcoidosis, lymphangitic spread of carcinoma or other neoplasms, and lymphoproliferative disease.
Note that a grouping of small subpleural nodules adjacent to the costal margins coexists. They are due to agglomerates of nodules and arranged linearly along the pleural surfaces, thus mimicking focal thickening (pseudoplaques ➨). Pseudoplaques are thought to be most commonly seen in association with granulomatous disease and in particular with sarcoidosis.
The distribution of the enlarged lymph nodes is a key sign for diagnosis of some diseases. Bilateral hilar lymph node enlargement may be a feature of infection (particularly fungal or mycobacterial infection) or malignancy (e.g., lymphoma), but sarcoidosis is the most common cause of bilateral lymph node enlargement.
Calcifications are often present in chronic sarcoidosis.
In our case the beaded septum sign, together with perilymphatic micronodules, pseudoplaques, and the symmetric lymphadenopathy with punctate calcifications are highly suggestive of sarcoidosis.
Management and Diagnosis
Transbronchial biopsy (TBB) can easily show non-necrotizing, well-formed granulomas, due to their centrilobular distribution (Figure A  ).
).
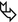 ).
).Transbronchial needle aspiration (TBNA) from mediastinal lymph nodes can also provide granulomas with similar features (please also refer to TBB and TBNA in the chapter Clinical Approach to DLD).
Final diagnosis: sarcoidosis

Pearls
Beaded septum sign consists of nodular thickening of interlobular septa reminiscent of a row of beads (Please see Figure B and Septal Pattern, subset Nodular). The beaded septum sign was initially described as a sign of lymphangitic spread of cancer, although thoracic sarcoidosis in the literature has been known as a “great mimicker” and can manifest with various pattern on HRCT, like nodular septal thickening simulating lymphangitic carcinomatosis.
Sarcoidosis, septal. Beaded septum sign often is an ancillary sign. It may be a predominant radiologic feature in only 15–20 % of patients with sarcoidosis. Other atypical manifestations of sarcoidosis, such as mass-like or alveolar opacities, honeycomb-like cysts, miliary opacities, mosaic attenuation, tracheobronchial involvement, and pleural disease, and complications such as aspergillomas may also be seen. The most common pattern in sarcoidosis, which helps to make a diagnosis, is the presence of micronodules with a perilymphatic distribution (avid of pleura) and bilateral, symmetric hilar lymph node enlargement.
Andreu J (2004) Septal thickening: HRCT findings and differential diagnosis. Curr Probl Diagn Radiol 33:226
Criado E (2010) Pulmonary sarcoidosis: typical and atypical manifestation at high-resolution CT with pathologic correlation. Radiographics 30:1567–1586
Bubble-Like Lucencies
Bubbly consolidation, pseudocavitations

Clinical History
Female in her 60s with asthenia, chronic dyspnea, arthralgia, and low-grade fever for about 2 months. Chest X-ray shows bilateral patchy consolidations. The picture remains unchanged after antibiotic therapy (“non-resolving pneumonia”).
HRCT
Patchy bilateral lung disease with alveolar pattern in the form of peripheral consolidations. All lesions show multiple bubble-like hyperlucencies ( ) and surrounding ground-glass opacity (halo sign
) and surrounding ground-glass opacity (halo sign 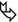 ).
).
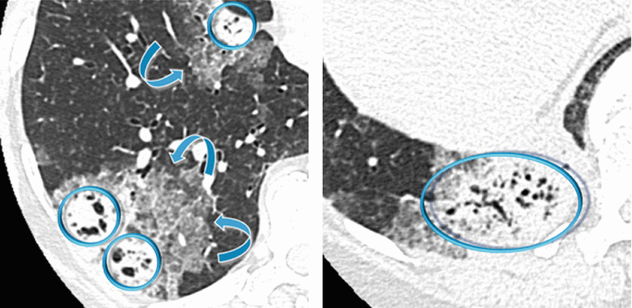
 ) and surrounding ground-glass opacity (halo sign
) and surrounding ground-glass opacity (halo sign 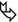 ).
).
Causes of Bubble-Like Lucencies
Neoplastic
Adenocarcinoma, primary or metastatic
BALT lymphoma
Nonneoplastic
Infection (e.g., TB), organizing pneumonia (OP), and pulmonary infarction
Tips and Tricks
The presence of hyperlucencies within a consolidation or within a subsolid nodule should not always be considered as a synonym of necrosis or cavitation. Small, rounded, or oval hyperlucencies, similar to bubbles (bubble-like lucencies), may be the expression of ectatic bronchioles (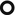 ); in this last case, therefore, they are pseudocavitations.
); in this last case, therefore, they are pseudocavitations.
The association between persistent opacities (“non-resolving pneumonia”) with bubble-like hyperlucencies and extended halo sign ( ) is suggestive of primary pulmonary adenocarcinoma, more frequently of the mucinous type.
) is suggestive of primary pulmonary adenocarcinoma, more frequently of the mucinous type.
Infectious disease (mainly, secondary tuberculosis), pulmonary infarction, organizing pneumonia (OP), and BALT lymphoma are other possible etiologies, more rarely encountered.
Management and Diagnosis
The patient underwent bronchoscopy. Bronchoalveolar lavage (BAL) showed increased lymphocyte and neutrophil count, absence of malignancy, and negative bacteriological examination and culture for mycobacteria. Histologic sampling by transbronchial biopsy (TBB, images below) revealed the presence of a small focus of adenocarcinoma; neoplastic cells grow along the alveolar septa (lepidic growth ►). They show a fence-like arrangement in a fence and show abundant intracytoplasmic mucin, with positive TTF-1 expression.
Final diagnosis: primary mucinous adenocarcinoma of the lung

Pearls
Bubble-like lucencies are small lucencies inside both nodules and consolidations. The origin of these pseudocavitations has been clarified by histopathological studies in patients with adenocarcinoma, in particular with the in situ mucinous adenocarcinoma (previously known as mucinous bronchoalveolar carcinoma or BAC).
Pathogenesis. Bubble-like lucencies (pseudocavitation) can be formed by a valve mechanism by bronchiolar obstruction or by desmoplastic bronchiolar traction or paracicatricial emphysema. Also, lucency can result from spared pulmonary lobules.
Lepidic growth is defined as cancer cells which proliferate respecting the microscopic structure of pulmonary alveoli and papering them like butterflies (Lepidoptera) on a fence.
Mucinous adenocarcinomas of the lung often manifest as diffuse lung involvement (multilobar and bilateral), showing patchy and extensive consolidations (pneumonia-like), often with bubble-like lucencies and possible halo sign. It also may display air bronchograms and the CT-angiogram sign, or it may be also associated with nodules, prevalently subsolid. It carries a worse prognosis than non-mucinous variants (survival by about 30 % at 5 years versus 70 %). Please also refer to adenocarcinoma in the Alveolar Diseases chapter.
Gaeta M (1999) Radiolucencies in bronchioloalveolar carcinoma: CT-pathologic correlation. Eur Radiol 9:55
Butterfly Sign
Bat’s wing/angel wing opacities

Clinical History
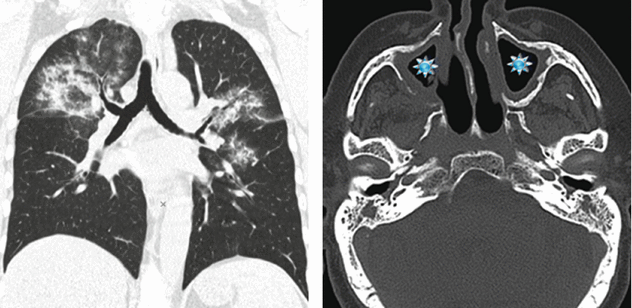
Woman in her 50s turned to the emergency room for sudden onset of hemoptysis and acute respiratory distress. The patient had also been suffering from low-grade fever and diffuse arthralgia for several months. At admission a chest radiography was performed, followed by a pulmonary HRCT study. A sinonasal CT was later performed due to upper airway symptoms.
CT
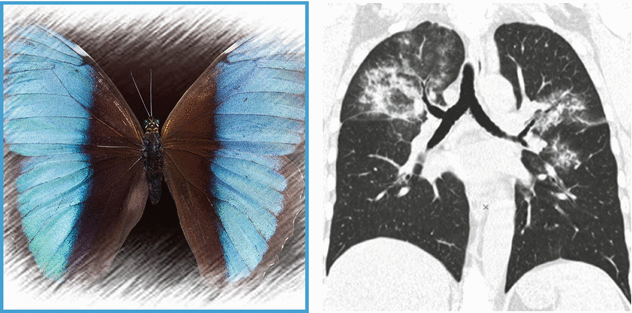
HRCT shows bilateral pulmonary involvement in the form of extensive ground-glass opacities. Distribution is central: note the presence of “subpleural sparing” (butterfly pulmonary opacities). CT scan of the paranasal sinuses shows bilateral maxillary sinus mucosal thickening ( ).
).
 ).
).
Causes of Butterfly Pulmonary Opacities
Acute
Hydrostatic pulmonary edema, diffuse alveolar hemorrhage, pneumocystis pneumonia, viralpneumonia, and aspiration or inhalation pneumonia
Chronic
Pulmonary alveolar proteinosis (PAP), adenocarcinoma, lipoid pneumonia (LP), “alveolar”sarcoidosis, and lymphoma/leukemia
Tips and Tricks
Diffuse parenchymal opacification, either with GGO or consolidations, constitute the alveolar pattern, which encompasses a wide range of diagnostic hypotheses. Consequently, it is very useful to consider the “time” factor: distinguishing acute from chronic opacification is very helpful in reducing the differential diagnoses (please see the tables at the end of Alveolar Pattern).
Bat’s wing distribution with acute symptoms primarily refers to a hydrostatic pulmonary edema. However, other acute lung diseases may show this distribution, such as diffuse alveolar hemorrhage (DAH), pneumonia, or inhalation/aspiration pneumonia.
The presence of Bat’s wing or butterfly pulmonary opacities without pleural effusion, and of other signs of pulmonary venous hypertension, makes the hypothesis of hydrostatic pulmonary edema unlikely.
Bat’s wing opacities in a patient with hemoptysis, acute respiratory distress, and signs of sinus inflammation support the final diagnosis of granulomatosis with polyangiitis (Wegener’s granulomatosis).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


