Gender-Related Differences in Lung Cancer
Silvia Novello
Julie R. Brahmer
Laura P. Stabile
Jill M. Siegfried
For several decades, lung cancer has been mainly viewed as a malignant disease affecting men; however, over the past 30 years, as a consequence of the dramatic increase in tobacco consumption in women, there has been an exponential increase in the incidence of the disease among women.1 Starting from 1990 to 1995, in many parts of the western world, the incidence of lung cancer in men has progressively declined as a consequence of antitobacco campaigns. If the worldwide sharp increase in women continues, the incidence of this malignancy is projected to be identical for women and men over the next decade.2 Overall, lung cancer causes the death of more women than the other three most common female cancers (breast, colorectal, and ovarian cancer) combined.
Sex differences in terms of susceptibility to carcinogens and natural history of the disease have been observed. When compared with men, women are more likely to develop adenocarcinoma and small cell carcinoma than squamous cell carcinoma, are more likely to be younger (<50 years old) at the time of diagnosis, are less likely to have a positive smoking history, and have a better survival at any stage (Table 25.1).3,4,5,6
EPIDEMIOLOGY
Internationally, lung cancer is the most often diagnosed cancer and is the leading cause of cancer-related deaths in men and women.7 In the United States, prostate cancer has the highest incidence in men, and in women, breast cancer has the highest incidence.8
Tobacco smoking became popular among women at the end of World War II, and the number of lung cancer cases and deaths in women started to increase around the 1960s and 1970s. The prevalence of smoking is extremely epidemic especially in high school girls: the percentage of American high school girls who smoke rose from 17.9% in 1991 to 27.7% in 2001.9
United States Data In the United States, the lung cancer incidence and mortality are different between men and women. The incidence rate is higher in men than in women regardless of age.3 In 2006, among new lung cancer cases, it has been estimated that 92,700 men (53% of the lung cancer cases) were diagnosed with lung cancer and 90,330 died of their disease. For women, 81,770 (47% of lung cancer cases) were diagnosed with lung cancer and 72,130 died of their disease.8 Lung cancer is the leading cause of cancer-related death in both sexes. Although the lung cancer incidence and mortality have been decreasing, since the early 1990s, in men, the incidence and mortality from lung cancer in women has been rising until just recently.10,11 The incidence in women peaked in 1991 at 33.1 per 100,000 person-years and has leveled off at 30.2 to 32.3 per 100,000 person-years between 1992 and 1999.3
Several other epidemiologic differences exist between the sexes. Utilizing the National Surveillance, Epidemiology, and End Results (SEER) database from 1975 to 1999, Fu et al.3 found that the median age at the time of diagnosis was 66 years for both men and women. However, more women were diagnosed at an age younger than 50 compared to men (8.6% vs. 6.9%, respectively; p = 0.0001). Overall incidence rates in both men and women younger than 50 years old have decreased. In those patients older than 50 years, the incidence of lung cancer decreased by 13.5% in men but increased by 37.3% in women from 1975 to 1987 and from 1988 to 1999. Lung cancer rates in women older than the age of 50 continue to rise.4,5,6,12,13 Among 228,572 patients with lung cancer registered in the SEER database, and diagnosed from 1975 to 1999, 35.8% were women. Women accounted for 40.9% of patients who were younger than 50 years of age.
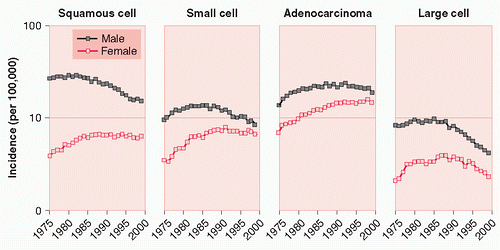
The proportional occurrence of histologic subtypes also differed significantly between men and women (p < 0.0001): in women, adenocarcinoma (44.7%) was the most common histologic subtypes, followed by small cell carcinoma (22.6%) and squamous cell carcinoma (21.4%).3 The incidence rate of adenocarcinoma increased in both men and women from 1975 to 1987, with an
increase seen in women of 40.5% versus 9.3% in men. In the same period, incidence rates for squamous cell and small cell carcinoma decreased in men, while increasing in women (Fig. 25.1). In the previously mentioned group of patients registered in SEER, the sex-related survival difference was greatest in patients with local disease and declined as the extent of disease increased.3
increase seen in women of 40.5% versus 9.3% in men. In the same period, incidence rates for squamous cell and small cell carcinoma decreased in men, while increasing in women (Fig. 25.1). In the previously mentioned group of patients registered in SEER, the sex-related survival difference was greatest in patients with local disease and declined as the extent of disease increased.3
TABLE 25.1 Sex Differences at Diagnosis in Different Studies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
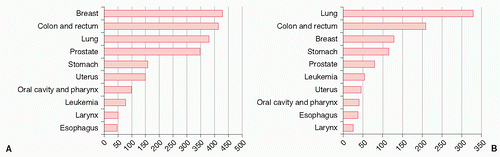
European Data Data from European cancer registries are slightly different than the U.S. statistics. In Europe, lung cancer accounts for 10% of cancer deaths in women compared to 28% in the United States.14 Lung cancer is not the most common cause of cancer-related deaths in women.15 Lung cancer ranks third behind breast and colon cancer in European women
(Fig. 25.2). Of course these data vary from country to country; the lung cancer-related deaths have increased in the United Kingdom and northern European countries, where lung cancer is now the leading cause of cancer-related deaths in women. The risk of lung cancer for women is highest in Denmark and Iceland. Data published from a Polish community-based cancer registry revealed that the age at diagnosis was younger for women compared to men (60.02 vs. 62.18 years, respectively; p < 0.001).4 Squamous cell cancer was the most common type of lung cancer in both sexes in Poland. Similarly to U.S. data, more women were diagnosed at an age younger than 50 years compared to men (23% vs. 12%, respectively). Also, women were more frequently nonsmokers (18.8%) than men (2.4%).
(Fig. 25.2). Of course these data vary from country to country; the lung cancer-related deaths have increased in the United Kingdom and northern European countries, where lung cancer is now the leading cause of cancer-related deaths in women. The risk of lung cancer for women is highest in Denmark and Iceland. Data published from a Polish community-based cancer registry revealed that the age at diagnosis was younger for women compared to men (60.02 vs. 62.18 years, respectively; p < 0.001).4 Squamous cell cancer was the most common type of lung cancer in both sexes in Poland. Similarly to U.S. data, more women were diagnosed at an age younger than 50 years compared to men (23% vs. 12%, respectively). Also, women were more frequently nonsmokers (18.8%) than men (2.4%).
Asian Data In Asia, the lung cancer mortality rates for women are lower than in the United States and Europe. However, the lung cancer mortality rates are increasing across several Asian countries including China, South Korea, and Japan.16,17,18 Adenocarcinoma tends to be the most common histology in women in Asia, and this proportion continues to increase over time.19,20,21,22,23,24 Although tobacco smoke is the most common cause of lung cancer in women throughout the rest of the world, the cause of lung cancer in the Asian woman is more complex. The proportion of female lung cancer patients who are never-smokers is 61% to 83%.25,26 In fact, only Filipino and Japanese women have a smoking rate higher than 10%.27 In comparison, the smoking prevalence is 22% in U.S. women.27 Environmental tobacco smoke and indoor pollutants, including cooking oil fumes and burning coal, have been implicated in increasing risks of lung cancer in nonsmoking Asian women.28,29,30,31,32,33,34,35,36,37,38,39
SUSCEPTIBILITY
Never-Smokers The risk of lung cancer is 2.5 times more common in female lifetime nonsmokers compared to male nonsmokers.40 Several studies have shown an association of increased risk of lung cancer, particularly adenocarcinoma, in never-smoking women with smoking husbands.41 Reasons for this are unclear; however, hormonal factors may play a role.42,43
Thun et al.44 investigated lung cancer death rates in lifelong nonsmokers. The age-standardized lung cancer death rate was 17.1 for men and 14.7 for women per 100,000 person-years. A small increase in death rate was seen between the periods of 1959 to 1972 and 1982 to 2000 in white and African American women, but not for men. The increase in death rate was only significant in women aged 70 to 84 years (p < 0.001).
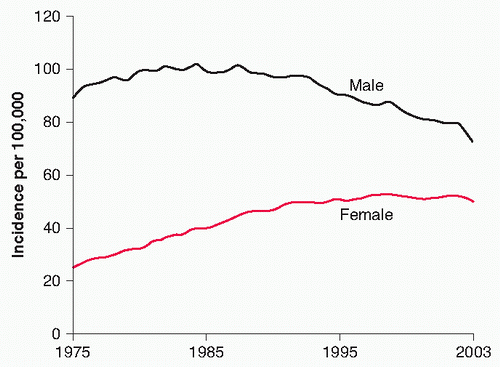
Smokers Epidemiologic studies demonstrate mixed results regarding female smokers and their susceptibility to develop lung cancer. Some studies note an increased risk of developing lung cancer in women compared to men (Fig. 25.3). Other
trials found similar risks between the sexes when controlling for smoking exposure.45,46,47,48 One trial concluded that smoking women had a 1.5-fold higher relative risk (RR) of lung cancer compared to men.49 Another study noted an odds ratio (OR) for developing lung cancer in a 40-pack-year smoker compared to a never-smoker of 27.9 in women versus 9.6 in men.50 In 2004, Henschke and Miettinen51 reported an increased risk of lung cancer of 2.7 (prevalence OR with 95% interval estimate of 1.6 to 4.7) in women versus men, controlled for age and smoking history, in a regression analysis done among smokers undergoing screening by computed tomography (CT) in the Early Lung Cancer Action Project.
trials found similar risks between the sexes when controlling for smoking exposure.45,46,47,48 One trial concluded that smoking women had a 1.5-fold higher relative risk (RR) of lung cancer compared to men.49 Another study noted an odds ratio (OR) for developing lung cancer in a 40-pack-year smoker compared to a never-smoker of 27.9 in women versus 9.6 in men.50 In 2004, Henschke and Miettinen51 reported an increased risk of lung cancer of 2.7 (prevalence OR with 95% interval estimate of 1.6 to 4.7) in women versus men, controlled for age and smoking history, in a regression analysis done among smokers undergoing screening by computed tomography (CT) in the Early Lung Cancer Action Project.
GENETIC/FAMILIAL FACTORS
Polymorphisms of genes that encode for enzymes, important in the breakdown of tobacco-derived carcinogens, may play a role in the development of lung cancer in female smokers and never-smokers. N-acetyltransferase (NAT2) activity can modify risk as well as cytochrome P450 (CYP) CYP1A2 activity (see Chapter 4). In Chinese female nonsmokers, low NAT2 activity and fast CYP1A2 activity had an adjusted OR of 6.9 compared to high NAT2 activity and slow CYP1A2 activity, making them at higher risk of developing lung cancer. Also, CYP1A1 is associated with an increased risk of lung cancer in female nonsmokers (OR = 3.97; 95% confidence interval [CI], 1.85 to 7.28).52 CYP1A1 is important in converting tobacco carcinogens into DNA-binding metabolites important in DNA adduct formation. Glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) are important for detoxification of carcinogens. GSTM1 null genotype is associated with an increased risk of lung cancer in some series but not in others.52,53,54,55 One study conducted among Japanese women demonstrated an association between GSTM1 null genotype and the increased risk of lung cancer particularly in lifetime nonsmoking women with the null genotype and the increased risk of lung cancer in never-smoker women with the null genotype and significant environmental tobacco smoke exposure (OR = 2.27; 95% CI, 1.13 to 2.7) compared with women without the null genotype and no significant environmental tobacco smoke (ETS) exposure.56 Similar to GSTM1, the null genotype of GSTT1 has shown an increased risk for the development of lung cancer in never-smokers.57
Nitadori et al.58 evaluated the association of lung cancer incidence and family history in the Japanese population. The study found that women had higher risks of lung cancer if a first-degree relative was diagnosed with lung cancer with a hazard ratio (HR) of 2.65 compared to similar men with an HR of 1.69. Other case-control studies found similar findings.59
VIRAL FACTORS
Human papillomavirus (HPV) may play a role in the development of lung cancer. In lung cancer, one study reported that female lung cancer patients who were never-smokers and older than 60 years of age had a higher prevalence of infection with HPV-16 and HPV-18.60,61 However, other studies have not demonstrated this same result in similar populations.62
DIET, RADON, OCCUPATIONAL EXPOSURES, AND PREEXISTING LUNG DISEASE
Sex differences have not been well studied regarding the risk of developing lung cancer related to occupational exposures independent of smoking (asbestosis, radiation, and other chemicals), diet, and radon. In one study of never-smoking women, researchers found that women exposed to asbestos (OR = 3.5; 95% CI, 1.2 to 10) and pesticides (OR = 2.4; 95% CI, 1.1 to 5.6) had an increased risk for developing lung cancer.63 Dry-cleaning workers were also found to have an elevated risk (OR = 1.8; 95% CI, 1.1 to 3.0). Exposure to radiation in the workplace or for treatment of other malignancies has been linked to the development of lung cancer. A recent study reported that breast cancer radiotherapy increased the risk of developing lung cancer particularly in smokers. Adjusted OR for smokers who received postmastectomy radiation was 18.9 (95% CI, 7.9 to 45.4). Nonsmokers in this study, who received postmastectomy radiation, did not have an increased risk for the development of lung cancer.64
Exposure to high levels of radon is also associated with an increased risk for developing lung cancer particularly in smokers
or those with exposure to secondhand smoke.65 Bonner et al.66 studied women pooled from several case-control studies that measured exposure to secondhand smoke and radon. The researchers also looked at the GSTM1 status of the person and found that in individuals exposed to residential radon who had GSTM1 null genotype, the risk of lung cancer was threefold higher than GSTM1 carriers (OR = 3.41; 95% CI, 1.10 to 10.61) even when adjusting for age, smoking status, and secondhand smoke exposure. In case-control and cohort studies, high dietary intake of fruits and vegetables decrease the risk of developing lung cancer.67 Of the fruits and vegetables studied, tomatoes and cruciferous have been associated with decreased risk for lung cancer.68,69,70
Other potential risk factors for lung cancer include preexisting lung diseases such as asthma and chronic obstructive pulmonary diseases. Several case-control studies demonstrate an increased risk for developing lung cancer in both men and women affected of these lung diseases.71 Even when controlling for active and passive tobacco exposure, some studies show an increased risk for lung cancer.72 Wu et al.73 studied nonsmoking women with previous lung disease and demonstrated an increased risk for lung cancer (adjusted OR = 1.56; 95% CI, 1.2 to 2.0).
STEROID HORMONES IN LUNG CANCER
Classical steroid hormone pathways have been successfully targeted in the treatment of breast and prostate cancer, where
hormone-dependent growth has been well established. Steroid hormone receptors are known to be expressed in tissues outside the reproductive tract and to have biological effects in nonre-productive tumors. Some effects mediated by steroid receptors appear to be independent of steroid ligands and result from activation of steroid receptors by phosphorylation pathways. Steroid hormone receptors could thus have biological activity via steroid-induced signaling or steroid-independent signaling. Because estrogen receptor (ER) signaling pathways that induce proliferation have been repeatedly found in non-small cell lung cancer (NSCLC), the ER is a promising target for lung cancer therapy. The progesterone receptor (PR) may play a role in lung cancer biology as well.
hormone-dependent growth has been well established. Steroid hormone receptors are known to be expressed in tissues outside the reproductive tract and to have biological effects in nonre-productive tumors. Some effects mediated by steroid receptors appear to be independent of steroid ligands and result from activation of steroid receptors by phosphorylation pathways. Steroid hormone receptors could thus have biological activity via steroid-induced signaling or steroid-independent signaling. Because estrogen receptor (ER) signaling pathways that induce proliferation have been repeatedly found in non-small cell lung cancer (NSCLC), the ER is a promising target for lung cancer therapy. The progesterone receptor (PR) may play a role in lung cancer biology as well.
ESTROGEN RECEPTORS IN LUNG CANCER
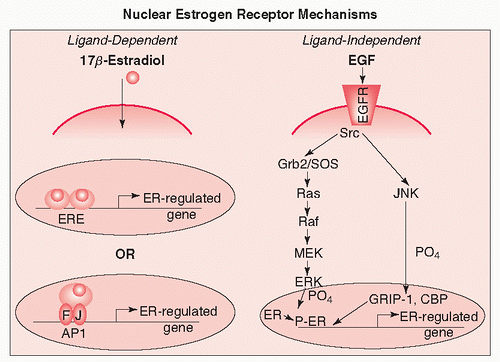
Studies of sex differences in lung cancer risk and disease presentation suggest that estrogens may be involved in the aetiology of this disease.74 For example, female patients are more likely to present with adenocarcinoma of the lung and to be never-smokers compared to male patients.75 As detailed previously, data have recently emerged that the rate of diagnosis of lung cancer in never-smoking women is higher than in never-smoking men.76 ERs, members of the nuclear steroid receptor superfamily, mediate cellular response to estrogen. Two forms of the ER have been identified, ER α and ER β, which are encoded by separate genes and display different tissue distributions. These proteins function either as ligand-activated transcription factors or can be activated by phosphorylation independent of ligand (Fig. 25.4).77
There have been inconsistent results reported concerning the presence of ERs in lung tumors. With the identification of antibodies that distinguish between ERα and ERβ andmore standard immunohistochemical procedures, it is now clear that ERβ is expressed and functional in most human NSCLC cell lines and is present in primary specimens of human NSCLCs from both men and women.78,79,80,81,82 There is less consensus on the expression of ERα in the lung. ERα was mainly found in the cytoplasm and membrane in immunohistochemical studies and was found to be comprised of mostly alternatively spliced variants based on immunoblot and RNA analysis.78 This nonnuclear ER α pool may be comprised of a variant isoform that lacks the amino-terminus, because it is differentially detected
by antibodies that recognize the ERα amino-and carboxy-terminal.79 ERβ, on the other hand, was found mainly localized to the nucleus with some cytoplasmic staining also observed and to be comprised of mainly full-length protein in addition to some variants.78 ER-mediated RNA transcription and proliferation in lung tumor cell lines support the hypothesis that at least some forms of ER are functional.78
by antibodies that recognize the ERα amino-and carboxy-terminal.79 ERβ, on the other hand, was found mainly localized to the nucleus with some cytoplasmic staining also observed and to be comprised of mainly full-length protein in addition to some variants.78 ER-mediated RNA transcription and proliferation in lung tumor cell lines support the hypothesis that at least some forms of ER are functional.78
Several reports relating ER status to NSCLC patient survival have been completed. Nuclear localization of ER β was observed in 45.8% to 69% of lung cancer cases79,80,81,82 and found to be a favorable prognostic indicator in all studies. In some cases, the prognostic significance was only observed in male patients. Nuclear ER α expression is either never detected or rarelydetected in NSCLC patient tumors.79,80,81,82,83 Prognostic significance of ERα was shown to have either no effect on survival or to correlate with poor prognosis.79,80 Kawai et al.79 reported that the presence of cytoplasmic ER α and the absence of ER β is associated with worse prognosis among NSCLC patients. Patients at higher risk at histopathologic stage I were those with no ER β expression.79 These results are opposite of what has been demonstrated for ER status and prognosis of breast cancer patients.84,85 Whether or not this relationship is observed in other patient populations is not known at the present time, and the specificity of some ER β antibodies has been disputed. Clearly, both nuclear and cytoplasmic ERs are important, and each component should be assessed separately and together, when examining patient tissue specimens for clinical evaluation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree