Identification of precursors of atrial fibrillation (AF) may lead to early detection and prevent associated morbidity and mortality. This study aimed to examine the association between frequent atrial premature complexes (APCs) and incidence of AF. For this retrospective cohort study, we analyzed Holter recordings obtained from 2000 to 2010 of 1,357 veterans free of AF at baseline. All pertinent data in electronic medical records were reviewed to ascertain baseline characteristics. Holter groups with frequent (≥100/day) and infrequent (<100/day) APCs were compared for development of new AF over a median follow-up of 7.5 years. Multivariate Cox regression analyses were performed before and after propensity score matching. Mean age was 64 years with 93% men. Mean body mass index, hemoglobin A1C, low-density lipoprotein, left atrial size, and heart rate were 31.24 kg/m 2 , 6.42%, 107.92 mg/dl, 4.26 cm, and 73 beats/min, respectively. AF was noted in 21.8% of patients with frequent APCs compared to 5.6% of those with infrequent APCs. After adjusting for demographics, medication use, co-morbidities, and laboratory and echocardiographic findings, multivariate Cox regression analyses confirmed frequent APCs to be independently associated with higher incidence of AF (hazard ratio [HR] 2.97, 95% confidence interval [CI] 1.85 to 4.80; p <0.001). In propensity-matched groups, this association remained significant (HR 2.87, 95% CI 1.65 to 4.98; p <0.001). Additionally, atrial couplets (≥50/day), atrial bigeminy (≥50/day), frequent runs of ≥3 APCs (≥20 runs/day), and longer runs (≥10 beats/run) were significantly associated with AF (HR 3.11, 3.67, 2.94, and 1.73, respectively, all p <0.05). In conclusion, frequent APCs (≥100/day) are associated with greater risk of AF.
Atrial fibrillation (AF) is the most common arrhythmia in the elderly. Its prevalence is estimated at 8.6% in Medicare beneficiaries and doubles with each advancing decade of life. With the median age of 64 years, veterans are at a significant risk. AF is an independent risk factor for stroke and mortality. It is responsible for recurrent hospital admissions and constitutes a significant health care burden. Treatment comprising rate and rhythm control strategies is fraught with medication-related adverse effects. Furthermore, prophylactic anticoagulant therapy puts this vulnerable elderly population at risk for bleeding complications. Early prediction and treatment of precursors could potentially reduce the incidence of AF and related morbidity, mortality, and health care costs. Atrial premature complexes (APCs) are commonly seen in healthy subjects. Although shown to trigger paroxysmal AF, they are, at present, considered innocuous and generally left untreated. Our hypothesis is that patients with frequent and recurrent APCs are more likely to develop AF.
Methods
VA Pacific Island Healthcare System Institutional Review Board and VA Central California Healthcare System Research and Development Committee approved this retrospective cohort study protocol. A database search was done to identify all veterans who had undergone Holter electrocardiographic (ECG) examination for any indication at VA Central California Healthcare System from 2000 to 2010. Patients thus identified were evaluated by chart review in electronic medical record. GE MARS 7.2 2005 software (GE Healthcare) was used to analyze the Holter data which were then verified by a VA staff cardiologist. Each report was systematically reviewed to evaluate for various parameters including predominant rhythm; minimum, maximum, and average heart rate; total beats; atrial and ventricular premature complexes including isolated beats, couplets, bigeminy, and beats in runs. Demographic information, co-morbid disease diagnoses, laboratory and echocardiographic data, and medication prescription information at the time of the Holter examination was manually collected by thorough chart review. Investigators crossverified data from randomly selected patients to ensure accuracy.
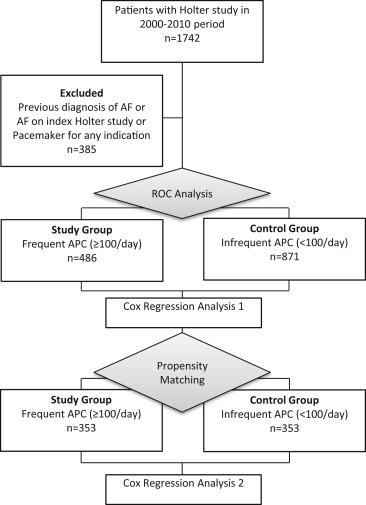
To identify patients free of AF at baseline, we reviewed problem lists, clinic notes, admission, progress and discharge notes, emergency room visits, and all available ECGs in the electronic medical records. Patients with a preexisting diagnosis of AF before the Holter ECG examination and those found to have new AF on the index Holter were excluded. Patients with atrial or ventricular pacing were excluded as well ( Figure 1 ).
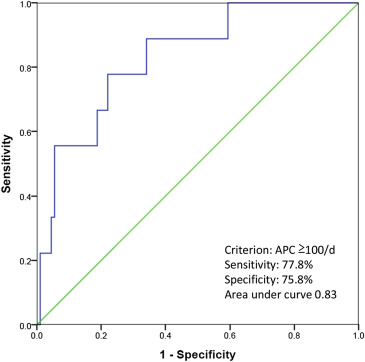
An atrial complex with <80% coupling interval to the preceding QRS, compared to the mean RR interval, was defined as APC. There exists no consensus definition of frequent APCs in previous reports. Previously defined cutoffs vary between studies. Therefore, a receiver operating characteristic curve analysis was performed on a random subset of the study population, with the aim of finding a reasonably accurate point of separation for APC frequency that will be most sensitive and specific to predict AF ( Figure 2 ). On the basis of the analysis, frequent APCs were defined as ≥100 events over a 24-hour ECG monitoring period. This cut-off number would yield a sensitivity and specificity of 77.8% and 75.8%, respectively, for predicting AF (area under curve = 0.83). Less than 100 APCs per 24 hours were defined as infrequent APCs. Frequent atrial couplets were defined as >50 couplets/day. Frequent runs of >3 APCs were defined as >20 runs/day. Long runs of APCs were defined as >10 beats per run.
Disease diagnoses of any given patient are enumerated in the “problem list” section of the VA electronic medical record system. Hypertension was defined by the problem list entry or any antihypertensive medication prescription. Diabetes mellitus was defined by the problem list entry, hemoglobin A1C ≥6.5%, or insulin or oral hypoglycemic agent use. Coronary artery disease was defined by problem list entry, history of coronary artery bypass surgery, or percutaneous coronary intervention. Problem list entries were used for the diagnosis of congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, obstructive sleep apnea, hypothyroidism, and hyperthyroidism. Moderate to severe left-sided stenotic or regurgitant valvular lesions were defined as valvular heart disease. Left atrial enlargement was defined as a left atrial diameter >3.8 cm in women or >4.0 cm in men.
Patients were grouped according to APC frequency as defined previously. Baseline characteristics of patients with and without frequent APCs were compared using independent t test or the chi-square test. Continuous variables were reported as mean ± standard deviation. Discrete variables were reported as number and percentage. Univariate regression analysis was conducted using known risk factors for AF (age, hypertension, diabetes, coronary disease, heart failure, left atrial enlargement, diastolic and systolic dysfunction) to evaluate their individual predictive value. Those characteristics found to be significantly associated with AF were added in a stepwise fashion to a Cox multivariate regression model to adjust for confounding.
After this analysis, a propensity score was derived with frequent APCs as the dependent variable and age, left atrial enlargement, and hypertension as covariates. Patients were then matched using an optimal matching method. Baseline characteristics of the matched groups were reevaluated. Cox proportional hazards regression method was used to compare the 2 groups with respect to the onset of AF. Data were censored at the time of AF identification or at the end of 13-year study period, whichever occurred first. Subsequently, subgroup analysis was done using the aforementioned methods to look predictive value of frequent isolated atrial complexes, frequent atrial couplets, frequent atrial bigeminy, frequent runs of ≥3 APCs, and long runs of APCs in AF in the propensity-matched cohort.
Hazard ratio (HR) and 95% confidence interval (CI) were used to depict strength of association. Two-tailed tests for statistical significance were reported, and p value of <0.05 was considered statistically significant. Kaplan–Meier estimator (not shown) was used for univariate and Cox proportional hazards modeling for multivariate survival analysis. Statistical calculations were performed using SPSS 20 (IBM SPSS Statistics for Windows version 20.0, Armonk, New York) and R Statistical Software version 2.13.2 (R Development Core Team, Vienna, Austria) with MatchIt package.
Comparison groups were followed through comprehensive electronic medical record review over a maximum follow-up of 13 years and a median follow-up of 7.5 years. Systematic review consisted of hospital admission and discharge summaries, emergency room visits, outpatient clinic notes, and all available electrocardiograms. The outcome of interest was incident AF.
Results
A total of 1,742 patients underwent Holter ECG examination for any indication from 2000 to 2010. After excluding 385 subjects with previously documented AF, baseline data were collected for 1,357 patients. Ninety-three percent of the study population was male with a mean age of 64 years. Twenty-two percent were smokers. Mean body mass index was 31.2 kg/m 2 , mean hemoglobin A1C was 6.4%, and mean low-density lipoprotein level was 107.9 mg/dl. Concomitant hypertension and diabetes mellitus were seen in 66% and 23% patients, respectively. Mean left atrial size was 4.2 cm, and average heart rate over 24-hour ECG recording period was 73 beats/min.
Table 1 summarizes baseline characteristics. Significant differences existed among comparison groups. Patients in the frequent-APC group were older with a mean age difference of 11 years. There were more men in the frequent-APC group. Racial differences were present as well. Patients with frequent APCs had a higher mean maximum heart rate and a lower mean minimum heart rate. Hypertension, coronary artery disease, heart failure, and obstructive pulmonary disease were more prevalent in the frequent-APC group. More left atrial enlargement and diastolic dysfunction were seen in the study group compared to the control group.
| Variable | Frequent APC | P value | Frequent APC After Propensity Score Matching | P value | ||
|---|---|---|---|---|---|---|
| Yes (n = 486) | No (n = 871) | Yes (n = 353) | No (n = 353) | |||
| Age, mean (years) | 71.4 ± 11.8 | 60.3 ± 13.4 | <0.001 | 67.9 ± 9.1 | 67.4 ± 8.9 | 0.41 |
| Men | 465 (95.7%) | 796 (91.4%) | 0.003 | 340 (96.7%) | 340 (96.7%) | 1.00 |
| Race | 0.04 | 0.21 | ||||
| White | 242 (49.8%) | 415 (47.6%) | 170 (48.2%) | 172 (48.7%) | ||
| Latino/Hispanic | 57 (11.5%) | 142 (16.3%) | 47 (13.3%) | 59 (16.7%) | ||
| Black | 20 (4.1%) | 60 (6.9%) | 13 (3.7%) | 22 (6.2%) | ||
| Asian | 3 (0.6%) | 8 (0.9%) | 2 (0.6%) | 3 (0.8%) | ||
| American Indian | 8 (1.6%) | 8 (0.9%) | 5 (1.4%) | 1 (0.3%) | ||
| Native Hawaiian | 3 (0.6%) | 4 (0.5%) | 2 (0.6%) | 2 (0.6%) | ||
| Unreported | 153 (31.5%) | 234 (26.9%) | 114 (32.3%) | 94 (26.6%) | ||
| BMI (kg/m 2 ) | 28.4 ± 5.7 | 29.3 ± 6.4 | 0.02 | 29.3 ± 5.8 | 27.3 ± 5.5 | <0.001 |
| Heart rate average (bpm) | 70.7 ± 12.5 | 74.0 ± 12.0 | <0.001 | 70.9 ± 12.7 | 71.5 ± 11.7 | 0.49 |
| Heart rate maximum (bpm) | 124.2 ± 65.7 | 116.9 ± 22.7 | 0.02 | 124.8 ± 75.6 | 111.6 ± 21.1 | 0.002 |
| Heart rate minimum (bpm) | 49.8 ± 12.7 | 52.0 ± 13.2 | 0.003 | 50.4 ± 13.2 | 51.5 ± 11.6 | 0.24 |
| Hypertension | 361 (74.3%) | 534 (61.3%) | <0.001 | 261 (73.9%) | 241 (68.3%) | 0.10 |
| Diabetes Mellitus | 109 (22.4%) | 198 (22.7%) | 0.90 | 84 (23.8%) | 89 (25.2%) | 0.66 |
| Coronary artery disease | 116 (23.9%) | 151 (17.3%) | 0.004 | 88 (24.9%) | 83 (23.5%) | 0.66 |
| Congestive heart failure | 34 (7.0%) | 35 (4.0%) | 0.02 | 21 (5.9%) | 20 (5.7%) | 0.87 |
| Chronic kidney disease | 27 (5.6%) | 34 (3.9%) | 0.16 | 18 (5.1%) | 18 (5.1%) | 1.00 |
| Chronic obstructive pulmonary disease | 66 (13.6%) | 69 (7.9%) | 0.001 | 44 (12.5%) | 40 (11.3%) | 0.64 |
| Obstructive sleep apnea | 14 (2.9%) | 27 (3.1%) | 0.82 | 11 (3.1%) | 8 (2.3%) | 0.49 |
| Hypothyroidism | 33 (6.8%) | 65 (7.5%) | 0.65 | 23 (6.5%) | 29 (8.2%) | 0.39 |
| Hyperthyroidism | 2 (0.4%) | 2 (0.2%) | 0.55 | 2 (0.6%) | 1 (0.3%) | 0.56 |
| Valvular Heart Disease | 15 (3.1%) | 23 (2.6%) | 0.63 | 9 (2.5%) | 17 (4.8%) | 0.11 |
| Habits | ||||||
| Smoking | 91 (18.7%) | 197 (22.6%) | 0.09 | 73 (20.7%) | 66 (18.7%) | 0.51 |
| Alcoholism | 53 (10.9%) | 132 (15.2%) | 0.03 | 41 (11.6%) | 48 (13.6%) | 0.43 |
| Illicit Drug use | 17 (3.5%) | 45 (5.2%) | 0.16 | 14 (4%) | 9 (2.5%) | 0.29 |
| Medications | ||||||
| Aspirin | 164 (33.7%) | 244 (28.0%) | 0.03 | 111 (31.4%) | 124 (35.1%) | 0.30 |
| Thienopyridines | 16 (3.3%) | 17 (2.0%) | 0.12 | 14 (4%) | 9 (2.5%) | 0.29 |
| Beta Blockers | 203 (41.8%) | 328 (37.7%) | 0.14 | 147 (41.6%) | 153 (43.3%) | 0.65 |
| CCBs | 73 (15.0%) | 96 (11.0%) | 0.03 | 52 (14.7%) | 43 (12.2%) | 0.32 |
| ACE Inhibitors | 183 (37.7%) | 257 (29.5%) | 0.002 | 142 (40.2%) | 121 (34.3%) | 0.10 |
| ARBs | 33 (6.8%) | 27 (3.1%) | 0.002 | 21 (5.9%) | 13 (3.7%) | 0.16 |
| Laboratory values | ||||||
| Cholesterol | 170.4 ± 38.2 | 185.1 ± 44.4 | <0.001 | 170.9 ± 37.6 | 177.8 ± 42.5 | 0.05 |
| Triglyceride | 134.0 ± 111.4 | 174.8 ± 209.0 | <0.001 | 142.8 ± 123.9 | 152.7 ± 129.8 | 0.37 |
| High-density lipoprotein | 42.0 ± 13.0 | 41.3 ± 12.9 | 0.45 | 40.6 ± 12.4 | 41.3 ± 11.2 | 0.47 |
| Low-density lipoprotein | 102.5 ± 33.6 | 111.3 ± 37.2 | <0.001 | 102.7 ± 32.9 | 106.6 ± 38.0 | 0.22 |
| Thyroid stimulating hormone | 2.1 ± 1.7 | 2.6 ± 8.4 | 0.22 | 1.9 ± 1.5 | 2.8 ± 9.1 | 0.20 |
| Hemoglobin A1C | 6.3 ± 1.1 | 6.5 ± 1.6 | 0.02 | 6.3 ± 1.2 | 6.6 ± 1.6 | 0.04 |
| ECHO parameters | ||||||
| Left Atrial Enlargement | 136 (28.0%) | 160 (18.4%) | <0.001 | 105 (29.7%) | 97 (27.5%) | 0.51 |
| Systolic Dysfunction | 0.48 | 0.29 | ||||
| Absent | 174 (79.8%) | 286 (82.9%) | 126 (79.7%) | 140 (83.3%) | ||
| Mild | 27 (12.4%) | 34 (9.9%) | 21 (13.3%) | 17 (10.1%) | ||
| Moderate | 7 (3.2%) | 15 (4.3%) | 3 (1.9%) | 7 (4.2%) | ||
| Severe | 10 (4.6%) | 10 (2.9%) | 8 (5.1%) | 4 (2.4%) | ||
| Diastolic Dysfunction | 132 (60.6%) | 173 (50.1%) | 0.02 | 90 (57%) | 95 (56.5%) | 0.94 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


