The prevalence of factors that are associated with an increased risk of stent thrombosis (ST), including smoking, diabetes mellitus, and small stent size, is different in women and men who underwent percutaneous coronary intervention. Thus, gender may potentially modify the relation between stent type and the incidence of ST during long-term follow-up. We explored the data of Patient Related Outcomes With Endeavor Versus Cypher stenting Trial (PROTECT) to evaluate this hypothesis. PROTECT randomized 2,061 women and 6,648 men who underwent percutaneous coronary intervention for various indications to Endeavor zotarolimus-eluting stenting (E-ZES) or Cypher sirolimus-eluting stenting (C-SES). Dual antiplatelet therapy was prescribed for at least 3 months. Data on study end points were collected until 5 years after randomization, including ST, death, and cardiovascular events. We analyzed end points and treatment effect (E-ZES vs C-SES) in relation to gender. Women were on average 4.7 years older (65.8 vs 61.1), had a higher prevalence of insulin-dependent diabetes mellitus, were less often smokers, and had a shorter total stent length than men. At discharge and throughout follow-up, a slightly lower fraction of women were using dual antiplatelet therapy. During 5-year follow-up, definite or probable ST was observed in 36 women (1.8%) and 152 men (2.4%; log-rank p = 0.15). E-ZES reduced the incidence of ST compared with C-SES in women (hazard ratio 0.58) and men (hazard ratio 0.61), with no evidence of heterogeneity (p = 0.89). In conclusion, in PROTECT, women and men had similar cumulative incidence of ST at 5 years after stent placement. The favorable effect of the study stent E-ZES over C-SES was not modified by gender.
Recently, a meta-analysis of randomized trials of patients who underwent percutaneous coronary intervention (PCI) showed that the use of newer generation drug-eluting stent (DES) is effective and safe in women during 3-year follow-up. However, the modifying effect of gender on clinical outcome after DES implantation, including stent thrombosis (ST), was not analyzed. Consequently, the interplay between gender, established risk factors for ST, dual antiplatelet therapy (DAPT) use, and patient outcome remains unclear. Against this background, we explored the data of the large (8,709 patients) Patient Related Outcomes With Endeavor Versus Cypher Stenting Trial (PROTECT) to evaluate the influence of gender on the incidence of ST (among other clinical end points) and on the relation between DES stent type and these end points during 5-year follow-up.
Methods
PROTECT is a prospective, open-label, multicenter, randomized, superiority trial, powered to study differences in long-term clinical effectiveness and safety in a broad group of coronary artery disease (CAD) patients with an indication for PCI. Details of the trial design and the main results have been published previously ( ClinicalTrials.gov , number NCT00476957 ). In short, 8,709 patients from 196 centers in 36 countries were randomized 1:1 to receive either an Endeavor zotarolimus-eluting stent (E-ZES; Medtronic CardioVascular, Santa Rosa, California) or a Cypher sirolimus-eluting stent (C-SES; Cordis, Johnson & Johnson, Warren, New Jersey) and otherwise were treated according to clinical practice. Patients aged ≥18 years who underwent elective, unplanned, or emergency procedures in native coronary arteries were eligible for enrollment if they provided written informed consent. The main exclusion criteria were a previous DES implantation at any time or a previous bare-metal stent implantation in the preceding 12 months, treatment with warfarin, or similar anticoagulant therapy. Enrollment started May 21, 2007, and was completed on December 22, 2008. The ethical committee of each participating center approved the study in accordance with local regulations.
The PCI and stent implantation technique was in accordance with the common clinical standards and the manufacturers’ instructions. Direct stenting was at the discretion of the operator. Staging of the procedures within 6 weeks of the initial procedure was allowed. DAPT therapy with aspirin and clopidogrel (75 mg) or ticlopidine was started 3 days before the procedure or through a loading dose for patients not yet taking these medications. Post-procedure, aspirin was to be given indefinitely and clopidogrel or ticlopidine therapy for a minimum of 3 months or up to 12 months, according to device instructions for use or guidelines. In accordance with clinical trial standards, investigators were allowed to extend the duration of DAPT or to restart thienopyridine therapy during the follow-up period if clinically indicated.
Study end points included the incidence of ST (definite or probable and definite), death (all-cause and cardiac), myocardial infarction (MI; major Q-wave MI and all nonfatal MI), and repeat coronary revascularization. ST was defined according to the Academic Research Consortium criteria and was subdivided in early (0- to 30-day post-stent implantation), late (>30 days), and very late (>12 months) ST. An independent Clinical Events Committee adjudicated ST, death, and MI after review of the original data sources. Revascularizations were site reported. For this analysis, we included end points up to 5-year follow-up.
Continuous variables are presented as both means ± SD and medians with interquartile ranges. Categorical variables are presented as counts and percentages. The distribution of baseline characteristics and clinical course variables were compared by gender using the Wilcoxon 2-sample test for continuous variables and the chi-square or Fisher’s exact test (in case an expected value in the corresponding contingency table was <5) for categorical data.
The incidences of the study end points are reported as Kaplan-Meier estimates. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored. Differences between women and men were evaluated using 2-sided log-rank tests.
Multivariate Cox proportional hazards models were used to obtain estimates of the relation between gender and study end points (and E-ZES vs C-SES treatment effect) that are adjusted for the broad range of clinical and angiographic factors that are listed in Tables 1 and 2 and assigned treatment, DAPT, gender × assigned treatment, and DAPT × assigned treatment (as applicable). Because the number of patients with possible or definite ST was limited, only factors with p <0.5 for that end point in univariate analysis were selected for multivariate adjustment. We report adjusted hazard ratios with corresponding 95% confidence intervals.
| Variable | Women (n=2061) | Men (n=6648) | P-value |
|---|---|---|---|
| Age (years) | 65.8 (10.0), 67 (59-73) | 61.1 (10.6), 61 (54-69) | <0.001 |
| Body-mass index (kg/m 2 ) | 28.0 (5.1), 27.5 (24.2-31.2) | 27.8 (4.3), 27.4 924.8-30.1) | 0.049 |
| Hypertension | 1506 (73.1%) | 4069 (61.2%) | <0.001 |
| Hyperlipidemia | 1373 (66.6%) | 4056 (61.0%) | <0.001 |
| Insulin dependent diabetes mellitus | 199 (9.7%) | 407 (6.1%) | <0.001 |
| Non-insulin dependent diabetes mellitus | 445 (21.6%) | 1360 (20.5%) | 0.28 |
| History of smoking | 778 (37.7%) | 4237 (63.7%) | <0.001 |
| Current smoker | 362 (17.6%) | 1820 (27.4%) | <0.001 |
| Family history premature coronary artery disease in first degree relative | 682 (38.3%) | 1918 (33.3%) | <0.001 |
| Prior myocardial infarction | 353 (17.1%) | 1439 (21.6%) | <0.001 |
| Prior percutaneous coronary intervention | 227 (11.0%) | 863 (13.0%) | 0.018 |
| Prior CABG | 71 (3.4%) | 352 (5.3%) | <0.001 |
| Prior heart failure | 71 (3.4%) | 194 (2.9%) | 0.24 |
| Prior peripheral vascular disease | 102 (4.9%) | 318 (4.8%) | 0.77 |
| Prior of stroke | 70 (3.4%) | 200 (3.0%) | 0.38 |
| Glomerular filtration rate (ml/min) | 79 (40%), 73 (56-93) | 96 (47%), 91 (73-113) | <0.001 |
| Women (n=2061) | Men (n=6648) | P-value | |
|---|---|---|---|
| Indication ∗ | <0.001 | ||
| Stable angina pectoris | 1014 (49.2%) | 3243 (48.8%) | |
| Unstable angina pectoris | 438 (21.3%) | 1200 (18.1%) | |
| ST-elevation myocardial infarction | 148 (7.2%) | 592 (8.9%) | |
| Non ST-elevation myocardial infarction | 364 (17.7%) | 1149 (17.3%) | |
| Silent ischemia | 97 (4.7%) | 464 (7.0%) | |
| Lesion location † | <0.001 | ||
| Left anterior descending coronary artery | 47.9% | 46.2% | |
| Right coronary artery | 31.6% | 28.9% | |
| Circumflex artery | 19.5% | 23.7% | |
| Lesion length (mm) † ‡ | 17.07 (8.94), 15 (10-20) | 17.88 (9.23), 15 (12-22) | <0.001 |
| Stenosis pre procedure (%) † ‡ | 81.98 (13.15), 74 (83-92) | 83.02 (12.76), 75 (85-92) | <0.001 |
| Minimal lumen diameter (mm) † ‡ | 0.52 (0.38), 0.5 (0.2-0.8) | 0.50 (0.38), 0.5 (0.2-0.7) | 0.038 |
| Reference vessel diameter (mm) † ‡ | 2.90 (0.46), 3.0 (2.5-3.1) | 2.99 (0.47), 3.0 (2.7-3.5) | <0.001 |
| Bifurcation † | 441 (15.6%) | 1574 (16.6) | 0.20 |
| Calcification | 0.25 | ||
| Non/mild | 2033 (72.0%) | 6695 (70.8%) | |
| Moderate | 616 (21.8%) | 2179 (23.0%) | |
| Severe | 176 (6.2%) | 588 (6.2%) | |
| Tortuosity | 0.16 | ||
| Non | 2207 (78.2%) | 7270 (76.8%) | |
| Moderate | 549 (19.4%) | 1964 (20.8%) | |
| Severe | 68 (6.2%) | 226 (2.4%) | |
| Number of lesions treated per patient ∗ | 0.032 | ||
| 0-1 | 1468 (72.1%) | 4585 (69.0%) | |
| 2 | 434 (21.1%) | 1508 (22.7%) | |
| 3 | 112 (5.4%) | 422 (6.3%) | |
| ≥4 | 29 (1.4%) | 130 (2.0%) | |
| Number of vessels treated per patient ∗ | 0.015 | ||
| 0-1 | 1705 (82.7%) | 5357 (80.6%) | |
| 2 | 333 (16.2%) | 1160 (17.5%) | |
| ≥3 | 23 (1.1%) | 128 (1.9%) | |
| Number of stents per patient ∗ | 0.032 | ||
| 0-1 | 1287 (62.4%) | 3927 (59.1%) | |
| 2 | 492 (23.9%) | 1733 (26.1%) | |
| 3 | 188 (9.1%) | 617 (9.3%) | |
| ≥4 | 94 (4.6%) | 368 (5.5%) | |
| Stent diameter (mm) § ‡ | 2.91 (0.40), 3.0 (2.5-3.0) | 3.00 (0.52), 3.0 (2.75-3.5) | <0.001 |
| Minimal stent diameter per patient (mm) ∗ ‡ | 2.86 (0.40), (2.75-2.50-3.00) | 2.93 (0.41), 3.00 (2.50-3.00) | <0.001 |
| Total stent length per patient (mm) ∗ ‡ | 29.52 (19.42), 24 (18-36) | 31.77 (21.15), 24 (18-41) | <0.001 |
| Staged procedure ∗ | 77 (3.7%) | 289 (4.3%) | 0.23 |
| Lesion success ¶ † | 2772 (99.5%) | 9265 (99.5%) | 0.99 |
| Procedure success ‖ ∗ | 1951 (96.3%) | 6328 (97.2%) | 0.038 |
† Per lesion; 2825 lesions in women, 9465 lesions in men.
‡ Mean (SD), median (interquartile range).
§ 3219 stents in women, 10,832 stents in men.
¶ Attainment of less than 50% residual stenosis of the target lesion with any percutaneous method.
‖ Attainment of less than 50% residual stenosis of all the target lesions and no inhospital major adverse cardiac events.
The age distribution was considerably different between women and men, whereas age was—not unexpectedly—related with the incidence of study end points. Hence, age was a major confounder of gender-outcome relations. Therefore, apart from applying multivariate analyses, we decided to report various results in 5 (clinically relevant) strata according to age.
A 2-sided p value <0.05 was considered statistically significant. Analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, North Carolina).
Results
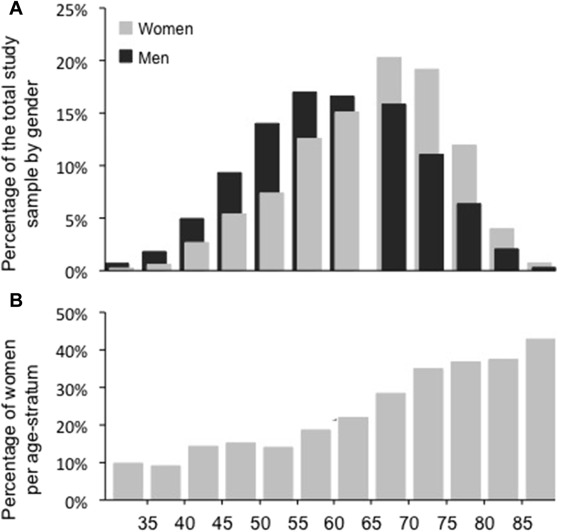
PROTECT enrolled 2,061 (23.7%) women and 6,648 men who underwent PCI ( Table 1 ). Women and men had a different age distribution ( Figure 1 ). Women were on average 4.7 years older (mean age 65.8 vs 61.1 year, p <0.001) and had a higher prevalence of traditional CAD risk factors, including hypertension (73.1% vs 61.2%, p <0.001), hyperlipidemia (66.6% vs 61.0%, p <0.001), and insulin-dependent diabetes mellitus (9.7% vs 6.1%, p <0.001) but a lower prevalence of smoking (37.8% vs 63.7%, p <0.001) and history of MI, PCI, or CABG. Likewise, women had a lower mean glomerular filtration rate (79 vs 96 ml/min, p <0.001) than men. A total of 53.9% women and 55.8% men underwent the index PCI for stable or silent angina, whereas the remaining patients were treated for acute coronary syndromes.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


