Laboratory testing is important for the safety of older adults initiating statins, but there has been little examination of laboratory testing disparities by race/ethnicity, age, gender, Medicaid eligibility, and multimorbidity. The study’s purpose was to examine disparities in guideline-concordant baseline laboratory testing and abnormal laboratory values among a retrospective cohort of 76,868 Medicare fee-for-service beneficiaries from 10 states in the eastern United States who had dyslipidemia and initiated a statin from July 1 to November 30, 2011. Guideline-concordant assessment of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was defined as evidence of an outpatient claim for either test within 180 days before or 14 days after the date of the index statin fill. In 2011, baseline laboratory testing rates were 89.3% for ALT and 88.8% for AST. Older adults were somewhat more likely to have ALT and AST testing if they were dually enrolled in Medicaid (relative risk 1.01, 95% confidence interval [CI] 1.00 to 1.02) or had multiple chronic conditions (relative risk 1.03, 95% CI 1.00 to 1.06 for 2 to 3 conditions; odds ratio [OR] 1.08, 95% CI 1.05 to 1.11 for 4 to 5 conditions; OR 1.14, 95% CI 1.11 to 1.17 for 6+ conditions), compared with 0 to 1 conditions. Non-Hispanic blacks were less likely to receive baseline testing (OR 0.97, 95% CI 0.96 to 0.98) than non-Hispanic Whites, and male beneficiaries were somewhat less likely to receive testing than female beneficiaries (OR 0.99, 95% CI 0.98 to 0.99). Abnormal values were rare. In conclusion, ALT and AST assessment after statin initiation was commonly done as recommended, and there were negligible disparities in testing rates for beneficiaries.
The purpose of this study was to examine rates of guideline-concordant baseline laboratory testing and disparities in testing for a cohort of Medicare fee-for-service (FFS) beneficiaries initiating statins in 2011. We also report the prevalence of abnormal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values at baseline, which have not been reported for Medicare FFS beneficiaries because of the historic lack of laboratory results data. We define at-risk groups to include beneficiaries who are racial and ethnic minorities, dually eligible for Medicare and Medicaid, older age, disabled, male, or have multiple chronic conditions.
Methods
The cohort of Medicare FFS beneficiaries examined in this study was obtained from a convenience sample of 10 eastern US states (New York, New Jersey, Maryland, Delaware, Virginia, North Carolina, South Carolina, Georgia, Florida, and Alabama), which were selected for a data linkage project described elsewhere. Medicare part B (i.e., non-hospital professional) claims for laboratory services were linked to actual outpatient laboratory values processed by a national laboratory vendor in 2011 for the Medicare FFS beneficiaries. Beneficiaries were retained if they were ≥65 years on January 1, 2011, were enrolled in parts A, B and D, the entire year (2011), were alive on December 31, 2011, initiated a statin, and had dyslipidemia using the co-morbid condition indicator in the beneficiary summary file ( Figure 1 ).
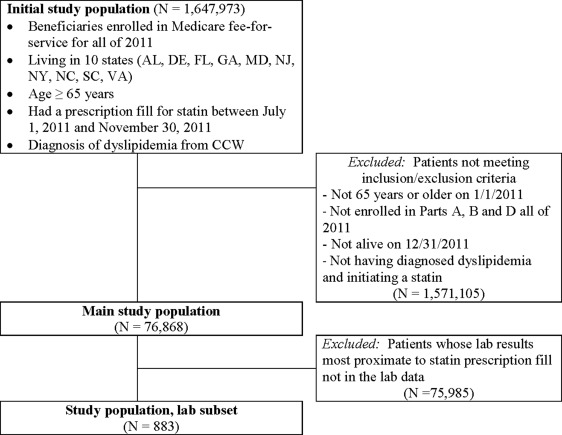
The Medicare part D events file was used to identify beneficiaries who initiated statins, based on a part D claim from July 1 to November 30, 2011, and no previous claims for a statin from January 1 to June 30, 2011. The date of the incident fill was defined as the index date. Statins were identified in the part D data by looking for any medications having the following generic drug names (amlodipine/atorvastatin, atorvastatin calcium, ezetimibe/simvastatin, fluvastatin sodium, lovastatin, niacin, lovastatin, niacin simvastatin, pitavastatin calcium, pravastatin sodium, rosuvastatin calcium, simvastatin, and sitagliptin/simvastatin). For analysis of test results values, we used data from the subset (n = 10,885 of 76,868) of these FFS beneficiaries who had AST and ALT values in the laboratory vendor records from the large national vendor, but there were an insufficient number of beneficiaries with abnormal values to examine disparities.
Consistent with previous analyses, we defined guideline-concordant baseline testing to have occurred if there was a part B claim for an ALT test within 180 days before the index fill or 14 days after the index fill. We identified ALT test using Current Procedural Terminology (CPT) codes 80,050, 80,053, 80,076, and 84,460 and AST test using CPT codes 80,050, 80,053, 80,076, and 84,450. We also defined separate indicators of guideline-concordant testing for each test (AST and ALT) and time frame (180 days before and 14 days after drug initiation) to examine whether guideline-concordant testing varied by clinical marker or time frame. Medicare data do not contain claims for inpatient laboratory testing, so beneficiaries who were hospitalized during the study period were credited with AST and ALT testing. Results were similar when beneficiaries were not credited with testing while hospitalized, so we present results that credit beneficiaries with testing while hospitalized.
Among the subset of beneficiaries who had a part B laboratory claim with a corresponding laboratory value, we examined the proportion with elevated ALT values (>120 units/L for men and >75 units/L for women) and elevated AST values (>120 units/L for men and >90 units/L for women) that would suggest baseline assessment and intensive monitoring is required.
Demographic characteristics and baseline comorbidities were summarized using medians with interquartile ranges for continuous variables and frequencies with percentages for categorical variables. We defined race/ethnicity (non-Hispanic White, Hispanic, non-Hispanic Black, and other), Medicaid dual eligibility, age groups (65 to 70, 71 to 75, 76+), eligibility (age vs disability), and gender from the Beneficiary Summary File. Multiple chronic conditions (0 to 1, 2 to 3, 4 to 5, and 6+) were constructed from the Chronic Condition Warehouse indicators for acquired hypothyroidism, atrial fibrillation, anemia, asthma, benign prostatic hyperplasia, cancer (as a combination of indicators for breast, colorectal, prostate, lung, and endometrial cancer), chronic kidney disease, chronic obstructive pulmonary disease, dementia/Alzheimer’s disease/related conditions, depression, diabetes mellitus, heart failure, hyperlipidemia, hypertension, ischemic heart disease, osteoporosis, rheumatoid arthritis/osteoarthritis, and stroke.
We present observed rates of baseline laboratory testing for AST or ALT and the combination of both tests by time period, overall, and by disparity group. To understand which Medicare FFS beneficiaries initiating statins were more likely or less likely to receive guideline-concordant laboratory assessment of both AST and ALT at baseline, we used log-binomial regression in SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, North Carolina) to estimate the association between optimal assessment and age (reference = age 65 to 70 years), gender (reference = female), race/ethnicity (reference = non-Hispanic Whites), Medicaid eligibility, eligibility (reference = age eligible) and multiple chronic conditions (reference = 0 to 1 co-morbidities). We reported 95% confidence intervals and used α = 0.05 to establish the statistical significance of all 2-sided tests. Among the subset with observed laboratory values, we examined the unadjusted proportion of beneficiaries with elevated AST or ALT. The institutional review board of the Duke University Health System approved the study.
Results
Demographic characteristics of the 76,868 Medicare FFS beneficiaries who had dyslipidemia and initiated statins from July 1 to November 30, 2011, are reported in Table 1 . The observed rates of guideline-concordant baseline laboratory testing in 2011 for ALT and AST testing were high ( Table 2 ) and primarily occurred in the 6 months before initiation. Unadjusted rates of guideline-concordant testing for both AST and ALT varied most widely by multiple chronic conditions, such that 91.1% of beneficiaries with 6+ conditions received baseline testing compared with 75.7% of beneficiaries with 0 to 1 conditions. Baseline testing rates were fairly similar across other patient characteristics.
| n = 76,868 | |
|---|---|
| Age (years) median (Q1, Q3) | 74.0 (70.0-80.0) |
| 65-70 | 22,856 (29.7%) |
| 71-75 | 19,940 (25.9%) |
| 76+ | 34,072 (44.3%) |
| Men | 28,674 (37.3%) |
| Hispanic | 5,724 (7.4%) |
| Non-Hispanic white | 59,656 (77.6%) |
| Non-Hispanic black | 8,692 (11.3%) |
| Other race/ethnicity | 2,796 (3.6%) |
| Eligibility criteria: | |
| Disability | 8,655 (11.3%) |
| Age | 68,213 (88.7%) |
| Medicaid enrolled | 16,067 (20.9%) |
| Atrial fibrillation | 8,156 (10.6%) |
| Dementia/Alzheimer’s/Related | 8,284 (10.8%) |
| Anemia | 27,455 (35.7%) |
| Asthma | 4,903 (6.4%) |
| Cancer | 8,390 (10.9%) |
| Chronic kidney disease | 14,093 (18.3%) |
| Chronic Obstructive Pulmonary Disease | 11,185 (14.6%) |
| Depression | 11,455 (14.9%) |
| Diabetes mellitus | 32,955 (42.9%) |
| Heart failure | 15,473 (20.1%) |
| Hypertension | 63,610 (82.8%) |
| Hyperlipidemia | 76,868 (100%) |
| Benign prostatic hyperplasia | 6,659 (8.7%) |
| Acquired hypothyroidism | 9,244 (12.0%) |
| Ischemic heart disease | 36,928 (48.0%) |
| Osteoporosis | 8,254 (10.7%) |
| Rheumatoid Arthritis/Osteoarthritis | 30,723 (40.0%) |
| Stroke | 4,695 (6.1%) |
| Number of Chronic Conditions | |
| 0-1 | 1,332 (1.7%) |
| 2-3 | 17,010 (22.1%) |
| 4-5 | 25,857 (33.6%) |
| 6+ | 32,669 (42.5%) |
| AST Testing | ALT Testing | Both Tests | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | 6 months Prior | 14 days After | Overall | 6 months Prior | 14 days After | Overall | 6 months Prior | 14 days After | |
| Overall | 68,223 (88.8%) | 67,052 (87.2%) | 8,436 (11.0%) | 68,653 (89.3%) | 67,502 (87.8%) | 8,465 (11.0%) | 65,890 (85.7%) | 64,699 (84.2%) | 8,032 (10.4%) |
| Age 65-70 | 19,959 (87.3%) | 19,578 (85.7%) | 2,132 (9.3%) | 20,111 (88.0%) | 19,734 (86.3%) | 2,137 (9.3%) | 19,191 (84.0%) | 18,807 (82.3%) | 2,014 (8.8%) |
| Age 71-75 | 17,679 (88.7%) | 17,367 (87.1%) | 2,094 (10.5%) | 17,770 (89.1%) | 17,460 (87.6%) | 2,105 (10.6%) | 17,036 (85.4%) | 16,714 (83.8%) | 2,012 (10.1%) |
| Age 76+ | 30,585 (89.8%) | 30,107 (88.4%) | 4,210 (12.4%) | 30,772 (90.3%) | 30,308 (89.0%) | 4,223 (12.4%) | 29,663 (87.1%) | 29,178 (85.6%) | 4,006 (11.8%) |
| Gender | |||||||||
| Female | 43,012 (89.2%) | 42,328 (87.8%) | 5,094 (10.6%) | 43,271 (89.8%) | 42,594 (88.4%) | 5,113 (10.6%) | 41,580 (86.3%) | 40,877 (84.8%) | 4,845 (10.1%) |
| Male | 25,211 (87.9%) | 24,724 (86.2%) | 3,342 (11.7%) | 25,382 (88.5%) | 24,908 (86.9%) | 3,352 (11.7%) | 24,310 (84.8%) | 23,822 (83.1%) | 3,187 (11.1%) |
| Ethnicity | |||||||||
| Hispanic | 5,234 (91.4%) | 5,116 (89.4%) | 699 (12.2%) | 5,239 (91.5%) | 5,120 (89.4%) | 701 (12.2%) | 5,142 (89.8%) | 5,021 (87.7%) | 679 (11.9%) |
| Non-Hispanic white | 52,934 (88.7%) | 52,059 (87.3%) | 6,426 (10.8%) | 53,310 (89.4%) | 52,455 (87.9%) | 6,451 (10.8%) | 50,951 (85.4%) | 50,067 (83.9%) | 6,096 (10.2%) |
| Non-Hispanic black | 7,562 (87.0%) | 7,433 (85.5%) | 1,029 (11.8%) | 7,611 (87.6%) | 7,483 (86.1%) | 1,028 (11.8%) | 7,356 (84.6%) | 7,219 (83.1%) | 982 (11.3%) |
| Others | 2,493 (89.2%) | 2,444 (87.4%) | 282 (10.1%) | 2,493 (89.2%) | 2,444 (87.4%) | 285 (10.2%) | 2,441 (87.3%) | 2,392 (85.6%) | 275 (9.8%) |
| Original eligibility | |||||||||
| Disability | 7,806 (90.2%) | 7,694 (88.9%) | 1,127 (13.0%) | 7,859 (90.8%) | 7,746 (89.5%) | 1,134 (13.1%) | 7,628 (88.1%) | 7,514 (86.8%) | 1,079 (12.5%) |
| Age | 60,417 (88.6%) | 59,358 (87.0%) | 7,309 (10.7%) | 60,794 (89.1%) | 59,756 (87.6%) | 7,331 (10.7%) | 58,262 (85.4%) | 57,185 (83.8%) | 6,953 (10.2%) |
| Medicaid enrolled | |||||||||
| No | 53,647 (88.2%) | 52,722 (86.7%) | 6,367 (10.5%) | 54,029 (88.9%) | 53,122 (87.4%) | 6,389 (10.5%) | 51,604 (84.9%) | 50,666 (83.3%) | 6,034 (9.9%) |
| Yes | 14,576 (90.7%) | 14,330 (89.2%) | 2,069 (12.9%) | 14,624 (91.0%) | 14,380 (89.5%) | 2,076 (12.9%) | 14,286 (88.9%) | 14,033 (87.3%) | 1,998 (12.4%) |
| Chronic Conditions | |||||||||
| 0-1 | 1,070 (80.3%) | 1,043 (78.3%) | 68 (5.1%) | 1,084 (81.4%) | 1,058 (79.4%) | 70 (5.3%) | 1,008 (75.7%) | 981 (73.6%) | 65 (4.9%) |
| 2-3 | 14,158 (83.2%) | 13,817 (81.2%) | 1,162 (6.8%) | 14,297 (84.1%) | 13,969 (82.1%) | 1,163 (6.8%) | 13,417 (78.9%) | 13,081 (76.9%) | 1,086 (6.4%) |
| 4-5 | 22,601 (87.4%) | 22,161 (85.7%) | 2,395 (9.3%) | 22,794 (88.2%) | 22,356 (86.5%) | 2,403 (9.3%) | 21,708 (84.0%) | 21,266 (82.2%) | 2,270 (8.8%) |
| 6+ | 30,394 (93.0%) | 30,031 (91.9%) | 4,811 (14.7%) | 30,478 (93.3%) | 30,119 (92.2%) | 4,829 (14.8%) | 29,757 (91.1%) | 29,371 (89.9%) | 4,611 (14.1%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


