Free Intestinal Transfer Techniques in Reconstruction of the Esophagus
Babak J. Mehrara
Joseph J. Disa
The advent of microsurgical techniques has revolutionized pha- ryngolaryngeal reconstruction. These techniques have enabled reconstructive surgeons to perform reliable single-stage repairs of complex defects with minimal morbidity and mortality. Microvascular free-flap reconstruction with intestinal conduits enables maintenance of oral secretions and rapid return of oral feeds and swallowing. Avoidance of dependency on tube feeds has a tremendous impact on quality of life and is particularly important in patients with limited long-term survival, such as those with locally advanced tumors of the pharyngoesophagus.
Microvascular transfer of intestinal tissues for esophageal reconstruction is indicated in the treatment of circumferential or partial defects of the esophagus resulting from surgical resection of pharyngoesophageal or laryngeal cancers, trauma, caustic stenosis, congenital esophageal atresia, congenital tracheo- esophageal fistula, and pharyngoesophageal stricture resulting from surgery or radiation therapy. Major reports have been published by Mansour26 and Carlson6 and their associates as well as by Coleman,9 Anthony and colleagues,1 and Chen and Tang.8 One of us (J.J.D.) and Cordiero13 and colleagues15 presented our own experience with these procedures. Although a number of vascularized intestinal conduits have been described and the ideal reconstructive technique is debated, the jejunum remains the most popular choice and is the workhorse in most centers.
History
Early surgical techniques for reconstruction of the cervical esophagus relied on placement of rigid stents or primary closure and were complicated by high rates of postoperative complications and mortality. Gastric pull-up procedures and pedicled colon or jejunal flaps were also described for reconstruction of the cervical esophagus; however, these procedures carried significant morbidity and mortality risks because of the need for extensive dissection in the abdomen and the chest and ischemic complications in the distal portions of the flap.
In the 1920s, Gilles and Blair popularized the use of random-patterned tubed skin flaps that were “waltzed” to the site of defect by picking up blood supply from the site of insertion. These procedures were multistaged and complicated; they commonly suffered from ischemic complications. Most patients had a controlled pharyngocutaneous fistula for 6 to 12 months, and many died prior to completion of the reconstruction. Wookey47 improved on these techniques by describing a laterally based tubed random cervical flap that decreased the time necessary for reconstruction. Although this technique was the standard of care until the mid-1960s, its use was limited owing to a relatively small amount of available skin (particularly above the hyoid bone), high rates of wound complications and fistula formation, and poor vascular supply after radical neck dissection or radiation therapy.
In 1965, Bakamjian4 revolutionized cervical esophageal reconstruction when he introduced the use of the deltopectoral flap. This flap, unlike other skin flaps used earlier, was based on an axial blood supply derived from perforating vessels arising from the internal mammary vessels through the second, third, and fourth intercostal spaces. This pattern of blood supply enabled reliable flap elevation that could be extended to produce large tube flaps if a delay procedure was utilized. The deltopectoral flap had the additional advantage of a remote blood supply that was not injured by radical neck dissection or previous radiation therapy. Using these flaps, Bakamjian4 reported a mortality rate of less than 4% and a fistula rate of approximately 30%. Although these results represented a significant advance in esophageal reconstruction, the use of the deltopectoral flap was still less than ideal because it was a multistage procedure (at least two stages if a delay was not necessary) with a controlled salivary fistula, and because the relatively poor blood supply of the distal portions of the flap did not improve wound healing in the neck.
Musculoskeletal flaps, in particular the pectoralis major myocutaneous flap (PMMF), were the next advance in cervical esophageal reconstruction because of their improved blood supply and the potential for single-stage reconstruction immediately after resection, as described by Ariyan.2,3 The rich blood supply of these flaps had the potential to improve wound healing in the neck and decreased fistula rates as compared with skin flaps, as noted by Ariyan3 and by Theogaraj and coworkers.44 The excessive bulk of the PMMF (particularly in women) complicated circumferential reconstruction in some patients and led to wound healing complications at the proximal esophageal anastomosis owing to the effect of gravity, making this reconstructive technique less than ideal.
In 1907, Alexis Carrel7 introduced the concept of microvascular tissue transfer by transferring a segment of jejunum to the cervical esophagus in a canine model. Technical difficulties, including the lack of adequate operating microscopes and microsurgical instruments, impeded the clinical use of these techniques until 1959, when Seidenberg and associates38 reported the first clinical case in which a free jejunum autograft was used to reconstruct a cervical esophageal defect in a patient with recurrent laryngeal cancer. The patient survived for 5 days but died suddenly of a cerebrovascular accident. Autopsy examination at that time, however, revealed that the flap was viable. In 1961, Roberts and Douglas35 reported the first successful case in which the patient was able to resume oral feeding and swallowing after microvascular jejunal transplantation. That same year, Hiebert and Cummings23 tubularized and transferred a segment of the gastric antrum to reconstruct the cervical esophagus and pharynx. The greater curvature of the stomach together with the greater omentum was first transferred by Baudet5 in 1979. This flap had the advantages of an extremely long vascular pedicle (>30 cm) and avoided the erosive complications associated with acid secretion by the transplanted gastric antrum. The sigmoid colon flap was reported by Nakayama32 and associates in 1962; however, concerns about abdominal and neck contamination kept interest in this flap relatively low.
Since the late 1970s, improvements in microsurgical techniques, instrumentation, and operating microscopes have increased the rates of success with free intestinal transfers, and these techniques have become the mainstay of cervical esophageal reconstruction. Because of the technical considerations, most centers rely primarily on the free jejunal flap for cervical esophagus reconstruction.
Microvascular Transplantation of the Jejunum
Indications and Contraindications
Microvascular transfer of the jejunum is indicated in the repair of circumferential and near-circumferential (50%) defects of the cervical esophagus. Although the jejunum free flap has been used as a patch for smaller defects of the cervical esophagus (<50%), we, as reported by one of us (J.J.D.) and colleagues,15 prefer to use the free radial forearm flap for these situations. Because of the segmental blood supply of the jejunum, up to 20 cm of jejunum can be harvested based on a single vascular arcade. In general, this length limits the use of the microvascular jejunal transfer to the cervical esophagus. Total and subtotal esophageal reconstructions extending to the thoracic esophagus are usually reconstructed with pedicled colon or gastric pull-up operations. If these options are not available, then reconstruction can be performed using a pedicled jejunal flap with distal revascularization in the neck or a free skin flap as described by Chen and Tang.8
Free jejunal transfer is contraindicated in patients with chronic intestinal diseases (e.g., Crohn’s disease), grave medical conditions, and chronic liver disease with ascites. The lack of available recipient vessels in the neck also precludes free jejunal transfer. Multiple previous abdominal operations with extensive adhesions are a relative contraindication.
Operative Technique
Before harvesting the jejunum, the reconstructive surgeon must ensure that recipient vessels in the neck are available for microsurgical transfer. These vessels should be in close proximity to each other, since it is not feasible to separate the mesenteric vein and artery widely. Arterial supply is reestablished by end-to-side anastomosis to the external carotid artery or end-to-end anastomosis to the lingual, facial, or superior thyroid arteries. The lingual artery should be used only if it is certain that the contralateral lingual artery is intact. Venous drainage is provided by end-to-side anastomosis to the internal jugular vein or end-to-end anastomosis to a suitable side branch. Vein grafts are avoided at all costs.
Preoperative bowel preparation and preoperative/intraoperative placement of enteral feeding tubes (percutaneous endoscopic gastrostomy, jejunostomy) are not routine, as noted by Flynn and Acland18 as well as by Fisher16,17 and Coleman10,11 and their colleagues. Broad-spectrum antibiotics are administered preoperatively, and Venodyne boots are placed prior to the induction of general anesthesia.
The jejunum is exposed by a midline upper abdominal incision. Although a vascularized segment of jejunum can be harvested from virtually anywhere between the ligament of Treitz and 150 cm beyond, a portion that is approximately 40 cm distal to the ligament is the usual location chosen, as recommended by McKee and Peters,28 McCaffrey and Fisher,27 and Shumrick and Savoury40 as well as by Salamoun36 and Wang46 and their associates. This is based on the fact that the pedicle length of this portion of the jejunum is longer than more proximal or distal portions. In addition, jejunal harvest from this portion of the intestine facilitates intra-abdominal bowel reanastomosis and enables the procurement of a second portion of jejunum without proximal ischemic complications if the first segment that was selected is lost owing to anastomotic thrombosis.
The bowel is transilluminated, and a suitable vascular arcade is selected (Fig. 143-1). Particular attention must be paid to the position of the recipient vessels in the neck in relation to the proximal bowel anastomosis with the cervical esophagus
(Fig. 143-2). The distance along a diagonal line from the proximal anastomosis to the position of the recipient vessels is carefully measured and is used to design the vascular pattern that will minimize kinking, stretching, or compression of the anastomosis (Fig. 143-3).
(Fig. 143-2). The distance along a diagonal line from the proximal anastomosis to the position of the recipient vessels is carefully measured and is used to design the vascular pattern that will minimize kinking, stretching, or compression of the anastomosis (Fig. 143-3).
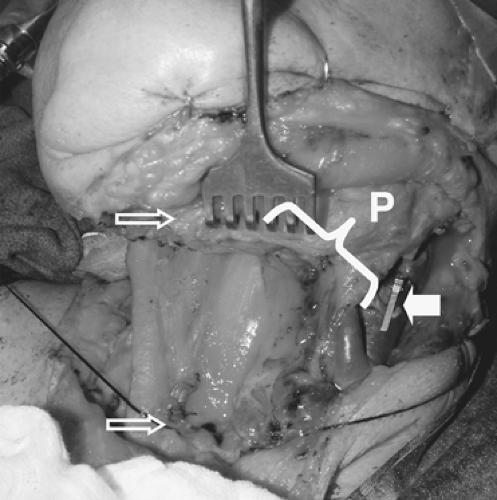 Figure 143-2. A typical cervical esophageal defect. In this case the defect spans between the open arrows. Note clip on lingual artery. Pedicle length (P) necessary in the jejunal flap is estimated by measuring the diagonal distance between the proximal esophageal stump and the location of the recipient vessels.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
