Fine-Needle Aspiration Cytology of Benign and Malignant Tumors of the Lung
Anjali Saqi
Madeline F. Vazquez
The cytologic diagnosis of lung cancer can be made by the evaluation of exfoliated cells in sputum and/or cells obtained by transbronchoscopic techniques including bronchial washing, bronchial brushing, bronchoalveolar lavage, and by fine-needle aspiration biopsy (FNAB), most commonly, transthoracic FNAB under computed tomography (CT) guidance. The sensitivity of a single sputum specimen for the detection of lung cancer is approximately 50% and increases with the number of specimens examined.1 The highest sensitivity is obtained for centrally localized squamous cell carcinoma and the lowest for small cell carcinoma. For endobronchial lesions that can be directly visualized with a bronchoscope, the sensitivity of a single bronchial washing or brushing for detecting lung cancer is about 65%, similar to endobronchial or transbronchial biopsy.2 Combining sputum cytology with bronchial brush cytology increases the sensitivity than with either method alone.3 Bronchoalveolar lavage utilizing narrow-diameter bronchoscopes has the higher yield for the detection of lung cancer in peripheral lesions with a reported sensitivity of 35% to 65%.4 By far, the most reliable cytologic method for diagnosing a localized peripheral lung lesion is transthoracic CT-guided FNAB, which has a sensitivity that exceeds 85% for the detection of lung cancer. Metaanalyses of transthoracic needle aspiration biopsy reports show a high sensitivity (0.88 to 0.99) and specificity (0.99 to 1.00) for the diagnosis of lung cancer.5 Most of the work on cytology screening for lung cancer has been on sputum specimens for the diagnosis of squamous cell carcinoma and its precursors. Lung cancer is thought to arise from a series of sequential preneoplastic changes that have been well defined for centrally arising squamous cell carcinoma but not other lung cancer subtypes. There are different patterns of molecular alterations in small cell carcinoma, squamous cell carcinoma, and adenocarcinoma. For example, Wistuba and colleagues6 found a higher incidence of deletions at 17p13 (TP53), 13q14 (RB), 9q21 (p16 INKa), 8p21-23, and several 3p regions in squamous cell carcinoma than in adenocarcinoma. On the other hand, K- RAS mutations have been detected in bronchoalveolar lavage fluids from patients with adenocarcinoma but not in patients with other lung cancer subtypes. It has been hypothesized that molecular analysis of sputum for biomarkers of lung cancer may provide an effective means of screening smokers to enable early lung cancer detection. However, the use of biomarkers for lung cancer screening is complicated by the fact that they can be detected in chronic smokers long before any clinical evidence of neoplasia. For example, p16 promotor hypermethylation and TP53 have been detected in sputum in chronic smokers without lung cancer and K- RAS mutations have been detected in atypical adenomatous hyperplasia (AAH).7 At our institution, the focus has shifted from the use of cytology for screening for early lung cancer to the diagnosis of CT screen-detected lung cancer by transthoracic FNAB. It is minimally invasive, does not require hospitalization, and serious risks are uncommon and limited to pneumothorax and minimal hemorrhage. The FNAB is performed using a 22-gauge Westcott needle with immediate on-site assessment of airdried smears utilizing the Diff-Quik staining method (Dade AG, Dudingen, Switzerland). Smears are also submerged in alcohol for Papanicolaou staining and blood clots are submitted directly in formalin for a cell block preparation.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma (Figs. 18.1 and 18.2) is strongly associated with cigarette smoking. It is a malignant epithelial tumor that was the most common histological subtype, but its incidence has now been surpassed by adenocarcinoma reflecting trends in reduced tobacco exposure with introduction of filters and low tar contents.8 Squamous cell carcinoma arises from dysplastic squamous epithelium and can present centrally or peripherally. In addition to the classic squamous cell carcinoma, the World Health Organization recognizes several additional histological patterns including papillary, clear cell, small cell, and basaloid variants.
Typically, squamous cell carcinomas are classified into well, moderately, and poorly differentiated categories, and the cytological appearance is dependent on the extent of differentiation.
In general, squamous cell carcinomas can be associated with tumor diathesis, acute inflammation, or foreign body-type giant cell reaction.9 In cytological preparations, squamous cell carcinoma occurs as single cells or loose clusters; tissue fragments are more common in aspirates.10 Nuclear:cytoplasmic ratios may range from low to high, with lower ratios being more frequent in the well-differentiated carcinomas.11 The cells have sharp distinct cell borders,11 and the nuclei are often hyperchromatic to pyknotic India ink with coarse chromatin.
In general, squamous cell carcinomas can be associated with tumor diathesis, acute inflammation, or foreign body-type giant cell reaction.9 In cytological preparations, squamous cell carcinoma occurs as single cells or loose clusters; tissue fragments are more common in aspirates.10 Nuclear:cytoplasmic ratios may range from low to high, with lower ratios being more frequent in the well-differentiated carcinomas.11 The cells have sharp distinct cell borders,11 and the nuclei are often hyperchromatic to pyknotic India ink with coarse chromatin.
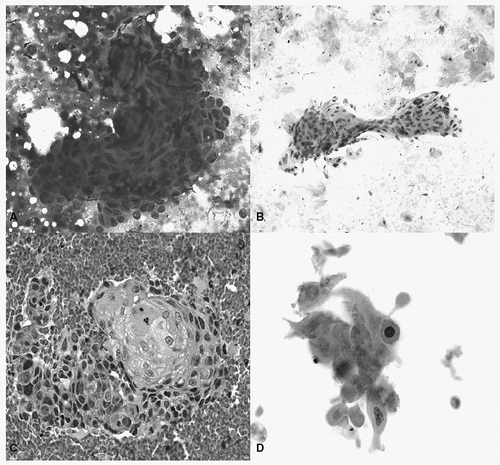 FIGURE 18.1 Squamous cell carcinoma. A: A syncytial cluster and rare single cells with high nuclear:cytoplasmic ratios and irregular nuclear borders (Diff-Quik, 40×). B: Orangeophilic cells present as clusters and singly with hyperchromatic nuclei and relatively low nuclear:cytoplasmic ratios; anucleate squames are also present (Papanicolaou stain, 20×). C: Cell block with intercellular bridges and keratinizing cells (cell block, hematoxylin and eosin [H&E], 40×). D: Polygonal-, spindle-, and bizarre-shaped squamous cells (Papanicolaou stain, 40×). (See color plate.) |
On histological sections, squamous cell carcinoma is defined by keratinization and/or intercellular bridges. In smears and liquid-based preparations of well-differentiated squamous cell carcinoma, intact intercellular bridges and keratin pearls are uncommon, 10 but they may be readily seen in cell
blocks sections. Rather, squamous cells are characterized by dense dark blue cytoplasm or orangeophilia on Diff-Quik and Papanicolaou-stained specimens, respectively. Polygonal cells, bizarre cells with tadpole/caudate and spindle cell configurations,10 and Herxheimer spirals in cytoplasmic tails are also associated with squamous differentiation. As squamous cell carcinoma becomes less differentiated, the aforementioned features become less prominent. Poorly differentiated carcinomas form syncytial groups especially in aspirates, and the cells have cyanophilic cytoplasm, higher nuclear:cytoplasmic ratios, and prominent nucleoli.9
blocks sections. Rather, squamous cells are characterized by dense dark blue cytoplasm or orangeophilia on Diff-Quik and Papanicolaou-stained specimens, respectively. Polygonal cells, bizarre cells with tadpole/caudate and spindle cell configurations,10 and Herxheimer spirals in cytoplasmic tails are also associated with squamous differentiation. As squamous cell carcinoma becomes less differentiated, the aforementioned features become less prominent. Poorly differentiated carcinomas form syncytial groups especially in aspirates, and the cells have cyanophilic cytoplasm, higher nuclear:cytoplasmic ratios, and prominent nucleoli.9
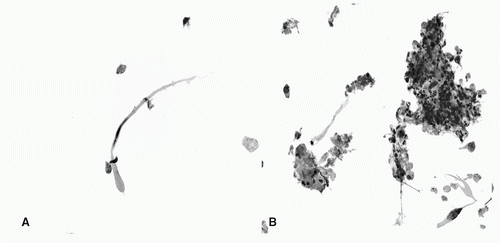 FIGURE 18.2 Squamous cell carcinoma. A: Atypical tadpole-shaped cell with hyperchromatic nucleus (Papanicolaou stain, ThinPrep, 40×) B: Marked acute infiammation and necrotic debris associated with squamous cells. Inset: malignant cells are also present (Papanicolaou stain, ThinPrep, 40× [inset: 60×]). (See color plate.) |
The remaining relatively uncommon variants of squamous cell carcinoma have predominantly been described in surgical pathology literature but awareness of these variants is important to preclude misdiagnosis. Histologically, basaloid squamous cell carcinoma shows cells with peripheral palisading, scant cytoplasm, and hyperchromatic nuclei admixed with areas of typical squamous differentiation.12 The small cell variant has focal squamous differentiation and small cells with morphologic traits of non-small cell carcinoma including coarse or vesicular chromatin, prominent nucleoli, and distinct cell borders.12,13
Carcinomas are often classified as non-small cell or small cell carcinoma for appropriate therapeutic management. An attempt to distinguish between squamous cell carcinoma and adenocarcinoma, rather than cataloging the two as poorly differentiated non-small cell carcinoma, is also becoming important as new agents for treatment are emerging. Bevacizumab, an antivascular endothelial growth factor monoclonal antibody, is being implemented for non-small cell lung cancer treatment, but it is contraindicated in patients with squamous cell carcinoma because of reports of pulmonary hemorrhage.14 Tyrosine kinase inhibitors of epidermal growth receptors are also being used to treat adenocarcinoma with bronchioloalveolar features likely being more sensitive than other non-small cell carcinomas.15 Given the differences in treatment options, subclassification of histological subtype can avert side effects as well as tailor appropriate therapy.
Immunohistochemistry Most squamous cell carcinomas express high-molecular weight cytokeratin, cytokeratins 5/6 and p63. Thyroid transcription factor-1 (TTF-1)16 and cytokeratin 7 (CK7)17 staining is present in a subset of cases.
Differential Diagnosis Cytology specimens with predominantly well-differentiated squamous cells have to be diagnosed cautiously. Such cells may represent mature keratinized superficial cells of a carcinoma without adequate sampling of smaller malignant cells, especially on exfoliative respiratory specimens,11 or they may reflect reactive, metaplastic, or degenerative changes, even in the presence of nuclear hyperchromasia and cellular irregularities.11 Reparative processes have atypia, but two-dimensional polarized “school of fish” sheets with enlarged nuclei, vesicular chromatin, and prominent nucleoli distinguish them from carcinoma.9 Atypical squamous cells can accompany marked acute inflammation and necrosis, suggestive of an abscess.11 An infectious process (e.g., aspergillosis) has to be considered, but intense orangeophilia and increased number of single cells should raise the possibility of squamous cell carcinoma.18 A histiocytic reaction to keratin can erroneously be interpreted as granulomatous inflamm ation.11
Basaloid and small cell variants of squamous cell carcinoma have similarities to (combined) small cell carcinoma.13 Cytological features, including dense cytoplasm, lack of nuclear molding, coarse chromatin, and nucleoli favor squamous cell carcinoma. Immunostains can aid in the diagnosis.
Finally, primary pulmonary squamous cell carcinoma is morphologically similar to its head and neck counterparts, and clinical history is necessary in determining the origin.
ADENOCARCINOMA
There has been a shift in the predominant histological subtype of non-small cell carcinoma. Adenocarcinoma (Figs. 18.3 and 18.4) has surpassed the incidence of squamous cell carcinoma, once the foremost culprit of lung carcinomas.8 Like squamous cell carcinoma, adenocarcinoma is linked to smoking, but it is also prevalent among nonsmokers and women.19,20 Adenocarcinoma, defined by glandular differentiation or mucin synthesis, tends to present peripherally. The World Health Organization classifies adenocarcinoma into acinar, papillary, bronchioloalveolar, solid with mucin production, and mixed patterns; less common variants such as fetal, mucinous cystadenocarcinoma, mucinous colloid, signet ring, and clear cell adenocarcinomas also comprise this category.12 Most adenocarcinomas demonstrate pattern heterogeneity and therefore are mixed subtype,21 whereas pure subtypes are comparatively infrequent.
The histological features of adenocarcinoma, including well, moderate, and poor differentiation, are often mirrored in cytology specimens. In general, adenocarcinoma has a clean or necrotic background,9 and has cyanophilic yet more translucent cytoplasm than squamous cell carcinoma.12 The cytoplasm may be foamy, granular, lacy, or vacuolated. Based on the underlying architecture and differentiation, the neoplastic cells are arranged as single cells, two-dimensional sheets, three-dimensional clusters, syncytial groups, acini, or papillae. Similarly, the cellular (cytoplasmic vacuolization) and nuclear features (intranuclear inclusions, intranuclear grooves, chromasia, chromatin pattern, and nucleoli) reflect the differentiation of the tumor.
Bronchioloalveolar Carcinoma Bronchioloalveolar carcinoma (BAC) is the in situ component of adenocarcinoma, which may be mucinous or nonmucinous. Histologically, BAC is defined as adenocarcinoma with predominantly lepidic growth along alveolar walls without stromal (desmoplastic reaction), vascular, or pleural invasion.12,22 Pure BAC is rather uncommon,23 but intermingling with other patterns, as in mixed subtype, is quite frequent. Among adenocarcinomas, BAC has a greater predilection among women24 and is more prevalent in nonsmokers. Because most BACs present peripherally, they are less likely to occur in sputum, lavages, and washes and are more amenable to aspiration biopsies. Relatively benign biological behavior and propensity for multifocality24 make identification of BAC significant rather than mere academic interest.
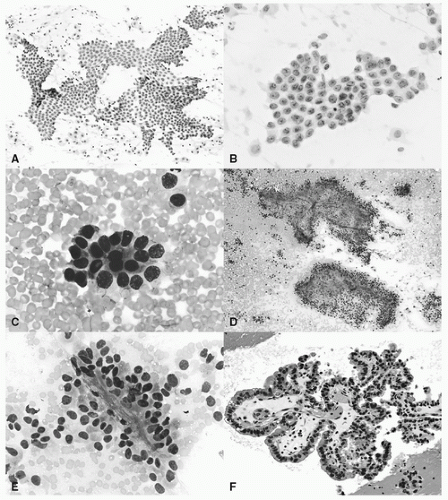 FIGURE 18.3 Adenocarcinoma. A: Monolayer sheets of relatively monotonous epithelial cells with pale chromatin and no significant nuclear overlap suggestive of BAC features (Papanicolaou stain, 20×). B: Two-dimensional sheet with bland nuclei containing pinpoint nucleoli and nuclear grooves often associated with BAC differentiation (Papanicolaou stain, 60×). C: Acinar formation (Diff-Quik, 20×). D: Fibrovascular cores surrounded by epithelial cells suggestive of papillary features (Diff-Quik, 10×). E: Epithelial cells enveloping delicate core in carcinoma with papillary architecture (Diff-Quik, 60×). F: Columnar cells line a fibrovascular core consistent with papillary features (cell block H&E section, 20×). (See color plate.) |
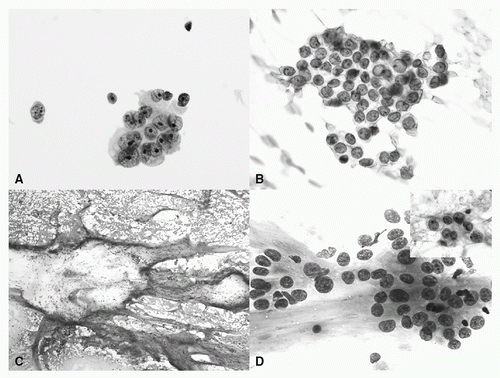 FIGURE 18.4 Adenocarcinoma. A: Three-dimensional cluster with nuclear pleomorphism, conspicuous nucleoli, and vacuolated cytoplasm (Papanicolaou stain, ThinPrep 60×). B: Cluster of epithelial cells with fine chromatin, inconspicuous nucleoli, intranuclear grooves, and inclusion (Papanicolaou stain, 60×). C: Adenocarcinoma with mucin: abundant mucin with scattered clusters of epithelial cells (Diff-Quik, 4×). D: Adenocarcinoma with mucin: malignant epithelial cells with conspicuous nucleoli and relatively abundant cytoplasm associated with mucin. Inset: Cells have vacuolated cytoplasm and nuclear membrane irregularities (Diff-Quik, 40×; inset Papanicolaou stain 60×). (See color plate.) |
Several studies have demonstrated BAC cytological and histological correlation.23,25,26,27 BACs tend to have a clean background, monolayered sheets 23 in orderly arrangement without nuclear overlap,26 single relatively uniform cells containing moderate to abundant cytoplasm, pale fine chromatin, and inconspicuous nuclei; intranuclear inclusions and invaginations may also be present.25 Most BACs are deceptively bland, but low-grade cytology should exclude neither a reactive process (over BAC) nor a well-differentiated invasive adenocarcinoma.27 Conversely, mild cellular pleomorphism is evident in BACs23 and does not imply invasive adenocarcinoma. Significant features distinguishing mucinous BAC from adenocarcinoma include abundant nuclear grooves, extracellular mucin,23 and greater propensity toward three-dimensional groups than sheets.28
Pure BAC or predominant BAC pattern in mixed adenocarcinoma is associated with better prognosis,26,29 so rendering a diagnosis of adenocarcinoma with BAC prominence on aspiration biopsy may guide therapeutic options. Lesions with a predominantly BAC may be amenable to relatively conservative surgical excision30 and preoperative testing for sensitivity to specific drugs.31,32 Detecting BAC on cytology also preoperatively alerts to the tendency of aerogenous spread and multifocal disease.26,33
Diagnostic accuracy of BAC is achieved by correlation with radiologic presentation (i.e., ground-glass opacity and solid component), operator expertise and adequate sampling. BAC features by cytology may yield predominantly non-BAC adenocarcinoma upon resection (or vice versa) as consequence of inadequately or peripherally sampled tumor.27 Although cytological examination can indicate BAC features, histological examination to unequivocally exclude invasion is necessary for definitive diagnosis.27
Adenocarcinoma In contrast to BAC, other subtypes of adenocarcinoma tend to have greater architectural complexity with three-dimensional clusters, cell balls, and syncytia. Presence of papillary fronds (often containing intranuclear inclusions, ± psammoma bodies34,26) and luminal/glandular formations echo histological papillary and acinar architectures, respectively. Micropapillary morphology, associated with aggressive behavior in breast, has also been described in the cytology literature.35 Cells of non-BAC tend to have conspicuous nucleoli,27 chromatin ranging from finely granular to coarse and hyperchromatic and minimal to marked pleomorphism in well to poorly differentiated adenocarcinomas, respectively. In poorly differentiated carcinomas, a non-small cell carcinoma rather than either squamous cell carcinoma or adenocarcinoma27 diagnosis is sometimes rendered. If available, material for immunostains to distinguish the two is beneficial as the tumors have different levels of sensitivity and adverse effects to therapeutic agents.
Immunohistochemistry Lung adenocarcinomas are frequently cytokeratin 7 (CK7)-positive and cytokeratin 20 (CK20)-negative. TTF-1,36 although specific for pulmonary (and thyroid) origin, is not 100% sensitive; incorporating thyroglobulin stain into the immunohistochemical panel, especially in presence of papillary architecture, is beneficial for excluding thyroid metastasis. Surfactant apoprotein, associated with lung origin, is also expressed in a fraction of nonpulmonary adenocarcinomas.37 Mucinous adenocarcinomas are often immunoreactive with CK7 and CK20, but not TTF-1.36
A CK7+, CK20−, TTF-1− immunoprofile in a lung mass is not uncommon. Because breast and upper gastrointestinal tract adenocarcinomas also exhibit this staining pattern, excluding metastatic disease is crucial. Inclusion of estrogen and progesterone receptors,38 gross cystic disease fluid protein, mammaglobin, and caudal-type homeobox transcription factor-2 (CDX-2) in the battery of stains is prudent in such cases. Estrogen/progesterone38 and CDX-239 expression have been also described in a subset of pulmonary adenocarcinomas.
Adenocarcinoma, BAC, and reactive atypia are morphological diagnosis. Although limited, evidence supporting application of p53 in discriminating BAC from non-BAC adenocarcinoma may have a role in cytology.28
Differential Diagnosis The main differential diagnosis problem is the distinction between peripheral adenocarcinoma of the lung and malignant pleural mesothelioma of epithelioid type. Malignant epithelioid mesothelioma has tubular and papillary architecture and CK7+/CK20−/TTF-1− profile like adenocarcinoma. Immunostains CD15, CEA, BerEP4 (for adenocarcinoma) and calretinin, CK 5/6, WT-1 (for mesothelial origin) can resolve the diagnosis of an epithelioid pleural-based lesion.
Several entities mimic adenocarcinoma including nonneoplastic lesions, metastases, mesothelial cells, and benign pulmonary neoplasms. Benign processes such as chemotherapy/radiation, pneumonia, and infarcts can mimic adenocarcinoma (see section on pitfalls). Reactive atypical pneumocytes can be challenging to differentiate from BAC on aspirates. Hyperplastic reactive cells display cilia and terminal bars. Also cellular enlargement, pleomorphism, binucleation, and nucleoli tend to be more prominent in reactive cells than BAC.27
Metastatic adenocarcinoma can appear histologically identical to pulmonary adenocarcinoma, and cytological features, although not unequivocally diagnostic, may steer in the distinction. Metastatic colon cancer is characterized by dirty necrosis and elongated nuclei. Primary lung adenocarcinoma often has mixed subtype with bronchioloalveolar features, whereas metastasis tends to illustrate morphological homogeneity. 12 Although these morphological features are helpful, confirmatory immunostains are necessary.
Sheets of mesothelial cells may result in misdiagnosis of BAC25; presence of intercellular bridges in mesothelial cells is helpful in separating the two entities.
Histologically, BAC is separated from its precursor AAH, with the latter measuring <5 mm and absence of interstitial
inflammation and fibrosis.12 In cytology, lesions that are suspicious but not diagnostic of adenocarcinoma, defined as scant clusters of bland, yet atypical bronchioloalveolar cells with uniform round nuclei, pinpoint nucleoli in a histiocytic background, are designated atypical bronchioloalveolar cell proliferation (ABP) and demonstrate BAC or invasive adenocarcinoma on resections,27 the discrepancy possibly a consequence of inadequate sampling.
inflammation and fibrosis.12 In cytology, lesions that are suspicious but not diagnostic of adenocarcinoma, defined as scant clusters of bland, yet atypical bronchioloalveolar cells with uniform round nuclei, pinpoint nucleoli in a histiocytic background, are designated atypical bronchioloalveolar cell proliferation (ABP) and demonstrate BAC or invasive adenocarcinoma on resections,27 the discrepancy possibly a consequence of inadequate sampling.
Sclerosing hemangioma and hamartoma are benign/low-grade pulmonary neoplastic mimickers of adenocarcinoma, and their differentiation from adenocarcinoma can alter surgical management. Sclerosing hemangioma is defined histologically by its constellation of architectural patterns including solid, papillary, hemorrhagic, and sclerotic and relatively bland stromal and surface cells.12 The architectural patterns of sclerosing hemangioma resemble those of adenocarcinoma, and the cells with bland cytology, inconspicuous nucleoli, intranuclear inclusions, and sparse/absent mitotic activity recapitulate features of BAC.40 Presence of hyalinized stromal tissue, foamy and/or hemosiderin-laden macrophages, two cell populations, and absence of marked pleomorphism are consistent with sclerosing hemangioma.40
Pulmonary hamartoma is a benign neoplasm composed of mesenchymal tissue (connective tissue, muscle, adipose tissue, and cartilage) with entrapped respiratory epithelium.12 The undifferentiated fibromyxoid component and bronchiolar cell proliferation with intranuclear inclusions and mild atypia resemble mucin and atypical cellular proliferation, respectively, suggestive of mucinous adenocarcinoma.41 Smears of the fibromyxoid stroma display fibrillar edges and entrapped spindle-shaped cells, whereas mucin is devoid of these characteristics; mildly atypical epithelial hyperplasia is described in hamartomas.41 Cartilaginous fragments and “popcorn calcification” on imaging are diagnostic of hamartoma.
NEUROENDOCRINE NEOPLASMS
Pulmonary neuroendocrine tumors comprise approximately 20% of lung malignancies.42 According to the most recent World Health Organization classification, they are histologically divided into a three-tier, four-category system including low-grade (typical carcinoid), intermediate-grade (atypical carcinoid), and high-grade (small cell carcinoma and large cell neuroendocrine carcinoma) tumors.22 Overall, all tumors have characteristic neuroendocrine architecture including organoid, nesting, trabecular, insular, palisading, ribbon, papillary, cord, or rosettelike patterns. The distinction among the spectrum of neuroendocrine tumors is largely based on the number of mitoses and presence/absence of necrosis. Typical carcinoids have less than two mitoses per 2 mm2 and no necrosis; atypical carcinoids have between 2 and 10 mitoses per 2 mm2 and/or necrosis; large cell neuroendocrine carcinomas and small cell carcinomas have greater than 10 mitoses per 2 mm2 and necrosis. Although the diagnostic criteria are primarily based on histological features, the architectural patterns and nuclear features are often reflected in cytological specimens. However, application of these criteria to neuroendocrine tumors in small biopsy specimens42 and cytological specimens can be challenging.
Typical and Atypical Carcinoids
Typical Carcinoid Typical carcinoid (Fig. 18.5) comprises approximately 1% to 2% of pulmonary neoplasms,42 and it is not associated with smoking. Typical carcinoid occurs at any age, with the mean age being 50 years.42 Carcinoid aspirates are usually hypercellular and demonstrate loose cell clusters and single cells.43 The cell groups often form syncytia, rosettes,43 and trabeculae. Clusters may also be associated with a prominent streaming, arborizing capillary network in a hemorrhagic background,44 a feature characteristic of typical and atypical carcinoids but not high-grade neuroendocrine carcinomas.45,46,47 Papillary architecture is also seen in some carcinoids.48,44 Single cells are also frequently seen in aspirates of carcinoids. Overall, the cells can have eccentrically placed nuclei with faint eosinophilic granular cytoplasm imparting a plasmacytoid appearance, or they may be stripped of their fragile cytoplasm and show bare nuclei.44 Nuclei are relatively uniform/monomorphic and round with smooth contours. Spindle-shaped cells are also present in spindle cell carcinoids. Slightly larger and pleomorphic cells can also be observed in carcinoids.47 The nuclei have fine granular chromatin and inconspicuous nucleoli. Rare intranuclear inclusions have also been described.44 No obvious nuclear molding, necrosis, or mitoses are observed. In addition to the aforementioned characteristics, carcinoids may also have oncocytic, acinic cell, signet ring cell, and melanocytic features, but these traits have no impact on prognosis.42
Atypical Carcinoids Atypical carcinoid (Fig. 18.6) patients have a mean age of 54 years42 and tend to be nonsmokers. Most of the cytology literature describing atypical carcinoid predates the current World Health Organization’s classification of neuroendocrine tumors. Despite the modification, the overall cytological features of atypical carcinoid are similar to typical carcinoids with few minor exceptions. The neoplastic cells may be slightly more pleomorphic and larger. In addition, atypical carcinoids are associated with mitoses and/or necrosis, similar to their histological counterparts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


