To date only sparse data are available on trends and changes in indications, patient’s characteristics, and clinical outcome of patients undergoing carotid artery stenting (CAS) in clinical practice. From February 1996 to December 2010, 6,116 CAS procedures were performed in 5,976 patients at 36 hospitals within the prospective, multicenter CAS registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte. Median age of patients was 71 years, 71.6% were men; a symptomatic stenosis was treated in 50.3% and an embolic protection device (EPD) was used in 82.5% of the patients. The overall hospital mortality or stroke rate was 3.1%. Stroke or in-hospital death occurred in 4.0% in symptomatic patients and in 2.2% in asymptomatic patients. In a logistic regression model, independent predictors of in-hospital death or stroke were heart failure (odds ratio [OR] 2.03, 95% confidence interval [CI] 1.22 to 3.36, p = 0.006), symptomatic stenosis (OR 1.52, 95% CI 1.05 to 2.18, p = 0.03), and age (OR per 10 years 1.46, 95% CI 1.17 to 1.81, p <0.001). The use of an EPD was significantly associated with a lower rate of death or stroke in the registry (OR 0.45, 95% CI 0.26 to 0.78, p = 0.004). From 1996 to 2010, mean age of patients increased by 4.1 years (p <0.001), the proportion of male patients decreased from 82.4% to 70.2% (p = 0.07), the proportion of symptomatic stenoses decreased (84.6% to 24.7%, p <0.001), and the use of EPDs increased from 1.4% to 97.2% (p <0.001). Comparing 2 periods from 1996 to 2003 and 2004 to 2010, a numeric decrease in the in-hospital stroke or death rate was seen in symptomatic (4.7% vs 3.5%, p = 0.11), and in asymptomatic patients (2.9% vs 2.1%, p = 0.27) undergoing CAS, which did not reach statistical significance. In conclusion, the proportion of symptomatic carotid artery stenoses decreased significantly; EPDs established as a standard tool and a numeric decrease of in-hospital stroke or death was seen in asymptomatic and symptomatic patients undergoing CAS in clinical practice over the last 15 years.
Carotid artery stenting (CAS) has been used an alternative treatment option of high-grade carotid artery disease over the last years in clinical practice. To determine the development of CAS over the last 15 years in an unselected population, we analyzed data of the prospective and multicenter CAS registry of the German Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK).
Methods
The CAS registry of the German ALKK was conducted from 1996 to December 2010 to document current indications, patient’s characteristics and outcome of CAS, as well as to improve quality of CAS by simple quality measurements in daily clinical practice. All interventions were prospectively enrolled in the registry and analyzed centrally. All complications occurring in the catheter room and during the in-hospital phase were prospectively documented. The registry has been described in detail previously. From 2011, all CAS interventions will be prospectively recorded in the “German Carotid Artery Stenting Registry” (GeCAS), which is a fusion of the ALKK CAS registry and the PRO-CAS registry (prospective registry of carotid angioplasty and stenting), which was conducted by the German Society of Angiology and the German Society of Radiology from 1999 to 2010.
For this analysis, all interventions from 1996 to the end of the ALKK CAS registry in 2010 were analyzed. To evaluate trends in CAS over the years, all interventions were categorized into 3 groups according to the date of the procedure (1996 to 2000, 2001 to 2005, and 2006 to 2011). Symptomatic patients had a history of ipsilateral stroke, at least 1 transient ischemic attack, or an episode of amaurosis fugax. In these patients, an angiographically documented stenosis ≥70% according to the diagnostic criteria of the North American Symptomatic Carotid Endarterectomy Trial was suggested to be an indication for an intervention. In case of asymptomatic patients, a stenosis ≥80% was suggested as an indication for intervention, especially in the presence of contralateral occlusion, before major surgery or documented progression of the stenosis. The decision to treat a patient was left to the treating physician. The risks and benefits of CAS were explained to the patients. Pts were informed about undergoing an investigational procedure and about the proved efficacy of CEA (carotid endarterectomy) in randomized trials. CEA was offered to every patient as an alternative treatment. Amaurosis fugax was defined as a retinal ischemia with transient monocular blindness. Transient ischemic attack was defined as a focal neurological deficit that resolved spontaneously within 24 hours. Stroke was merely a clinical diagnose, which was defined as loss of neurological function caused by an ischemic or hemorrhagic event with residual symptoms at least 24 hours after onset; a minor stroke was diagnosed if symptoms disappeared within 1 week after onset and a major stroke was diagnosed if symptoms persisted for at least >1 week after onset. There was no routine recording of the results of cranial computed tomography or magnetic resonance imaging scan after CAS from 1996 to 2008. Consequently, no differentiation between ischemic stroke, hemorrhagic stroke, and stroke with unknown cause was made until 2008. From January 2008 to December 2010, these variables were recorded and evaluated separately. No quantification of the severity of stroke (such as the National Institute of Health classification), besides its reversibility, was made. End points were evaluated by a neurologist either immediately at the occurrence of symptoms or routinely at the end of the hospital stay. Independent neuroassessment was performed in 73.9% of all patients at hospital admission and in 59.3% of all patients at hospital discharge. We did not separately evaluate 30-day follow-up end points until 2008. Since then, 30-day event rates were collected prospectively. We also attempt to achieve a follow-up of patients treated before 2008 to determine 30-day event rates, which were calculated by the Kaplan-Meier method. Completeness of these data before 2008 was achieved in 66% of all enrolled patients. The combined clinical end point of “all death and strokes” was prospectively defined as the primary end point of the registry. Different angiographic appearances of the stenosis were defined as follows: (1) ulcer: an excavation at the site of the stenosis and (2) visible thrombus: a round-shaped filling defect at the stenosis site, either mobile or not mobile. Quantification of the severity of the stenosis was performed using the North American Symptomatic Carotid Endarterectomy Trial method. The use of a quantitative angiographic measurement or just “eye-balling” was left to the discretion of the treating physician. The CAS procedure was performed according to the standard protocol of each participating center. The patient population is described by absolute numbers and percentages. The distribution of continuous variables is characterized by median and quartiles. The group of patients experiencing death or stroke during hospital stay was compared with the group not experiencing this end point using the Pearson-Fisher chi-square test with regard to binary variables and the Wilcoxon-Mann-Whitney test with regard to metrical variables. The 2-tailed Cochran-Armitage test was used to analyze changes over the years. Predictors for the occurrence of in-hospital death or stroke were analyzed in a logistic regression model. The baseline characteristics that showed a significant association with the end point in univariate comparisons were included in a backward selection removing variables with p >0.2. For each explanatory variable, adjusted odds ratios (ORs) with 95% confidence limits were computed. The discrimination of the model was assessed by the C statistic. p values ≤0.05 were considered significant. All p values are results of 2-tailed tests. The statistical computations were performed using the SAS statistical package, version 9.3 (SAS Institute, Cary, North Carolina).
Results
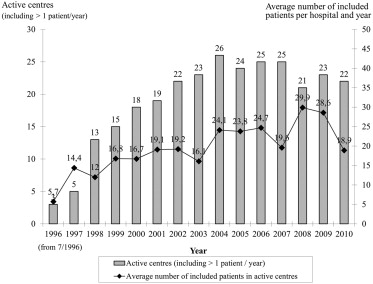
From February 1996 to December 2010, 5,976 patients underwent 6,116 interventions at 36 hospitals and were enrolled into the prospective ALKK CAS registry. The numbers of enrolled patients in the registry and the numbers of participating hospitals over the years are shown in Figure 1 . The median postinterventional in-hospital stay averaged 2 days (quartiles 2 to 4 days).

Baseline characteristics, concomitant diseases, and interventional characteristics of the total population included in the registry from 1997 to 2010 are listed in Table 1 . Mean age of the total population was 71 years (quartiles 65 to 77 years). A total of 71.6% of all patients in the registry were men and symptomatic carotid stenoses were treated in 50.3% of all patients in the total population. Many patients suffered from concomitant diseases like coronary artery disease (65.5%), previous myocardial infarction (25.8%), and peripheral arterial disease (25.1%). The intended carotid intervention was performed in 97.2% of all procedures with a rate of 97.6% stent implantation. In 2.8% of all procedures, the intervention was interrupted before balloon dilatation mainly because of vascular access problems or impossibility of passing high-grade stenoses with the guidewire. Median procedural time of CAS averaged 40 minutes (quartiles 27 to 53 minutes). An embolic protection device (EPD) was used in 82.5% of all performed CAS procedures in the registry.
| Patients Interventions | Total (n=5976) (n=6116) | Death or Stroke | p-value | |
|---|---|---|---|---|
| Yes (n = 186) (n = 191) | No (n = 5790) (n = 5925) | |||
| Age (years) | 71 (65-77) | 75 (68–79) | 71 (65-77) | <0.01 |
| Symptomatic carotid stenosis | 2817/5598 (50.3%) | 113/174 (64.9%) | 2704/5424 (49.9%) | <0.01 |
| Coronary heart disease | 3878/5923 (65.5%) | 124/186 (66.7%) | 3754/5737 (65.4%) | 0.73 |
| Prior myocardial infarction | 1531/5927 (25.8%) | 54/184 (29.3%) | 1477/5743 (25.7%) | 0.27 |
| Peripheral arterial disease | 1479/5893 (25.1%) | 50/183 (27.3%) | 1429/5710 (25.0%) | 0.48 |
| Heart failure (≥ NYHA class III) | 403/4817 (8.3%) | 22/140 (15.7%) | 381/4731 (8.1%) | <0.01 |
| Atrial fibrillation | 506/4901 (10.3%) | 21/141 (14.9%) | 485/4760 (10.2%) | 0.07 |
| Severe Chronic obstructive pulmonary disease | 232/4900 (4.7%) | 9/141 (6.4%) | 223/4759 (4.7%) | 0.35 |
| Arterial hypertension | 4526/4935 (91.7%) | 135/144 (93.8%) | 4391/4791 (91.7%) | 0.37 |
| Diabetes mellitus | 1660/4904 (33.8%) | 60/143 (42.0%) | 1600/4761 (33.6%) | 0.04 |
| Hypercholesterolemia # | 4100/4921 (83.3%) | 118/144 (81.9%) | 3982/4777 (83.4%) | 0.65 |
| Current smoker | 1404/4884 (28.7%) | 31/141 (22.0%) | 1373/4743 (28.9%) | 0.07 |
| Renal impairment | 501/3091 (16.2%) | 19/84 (22.6%) | 482/3007 (16.0%) | 0.11 |
| Ipsilateral symptoms | 2161/5235 (41.3%) | 81/158 (51.3%) | 2080/5077 (41.0%) | 0.01 |
| Prior ipsilateral TIA | 951/2113 (45%) | 36/81 (44.4%) | 915/2032 (45%) | 0.92 |
| Prior ipsilateral minor stroke | 372/2113 (17.6%) | 15/81 (18.5%) | 357/2032 (17.6%) | 0.83 |
| Prior ipsilateral major stroke | 514/2113 (24.3%) | 25/81 (30.9%) | 489/2032 (24.1) | 0.16 |
| Contralateral symptoms | 293/5235 (5.6%) | 16/158 (10.1%) | 277/5077 (5.5%) | 0.01 |
| Prior ipsilateral carotid intervention | 356/5927 (6.0%) | 8/183 (4.4%) | 348/5744 (6.1%) | 0.34 |
| Interventional Characteristics | ||||
| Procedural time (minutes) | 40 (27-53) | 50 (35-65) | 39 (27-53) | <0.01 |
| Carotid intervention performed | 5898/6069 (97.2%) | 181/189 (95.8%) | 5717/5880 (97.2%) | 0.23 |
| Stent implanted during procedure | 5759/5900 (97.6%) | 175/180 (97.2%) | 5584/5720 (97.6%) | 0.73 |
| Recurrent stenosis treated | 297/4878 (6.1%) | 6/140 (4.3%) | 291/4738 (6.1%) | 0.37 |
| Visible thrombus in stenosis | 360/5896 (6.1%) | 20/186 (10.8%) | 340/5710 (6.0%) | <0.01 |
| Length of stenosis > 10mm | 2029/5896 (34.4%) | 82/186 (44.1%) | 1947/5710 (34.1%) | <0.01 |
| Maximal grade of stenosis previous to procedure [%] | 86.4±14.2 | 86.7±9.3 | 86.4±14.3 | 0.41 |
| Maximal grade of stenosis post procedure [%] | 7.4±12.9 | 10.1±17.5 | 7.3±12.8 | 0.05 |
| Predilatation performed | 1134/2027 (55.9%) | 39/56 (69.6%) | 1095/1971 (55.6%) | 0.04 |
| Postdilatation performed | 1834/1964 (93.4%) | 45/46 (97.8%) | 1789/1918 (93.3%) | 0.36 |
| Protection device used | 4821/5842 (82.5%) | 124/180 (68.9%) | 4697/5662 (83%) | <0.01 |
| Medication at hospital discharge | ||||
| Aspirin | 5644/5898 (95.7%) | 143/152 (94.1%) | 5501/5746 (95.7%) | 0.32 |
| Clopidogrel | 5438/5623 (96.7%) | 124/134 (92.5%) | 5314/5489 (96.8%) | 0.01 |
| Vitamin K antagonist | 319/5826 (5.5%) | 6/152 (3.9%) | 313/5674 (5.5%) | 0.40 |
| ACE inhibitor/AT1-antagonist | 3347/4852 (69.0%) | 76/111 (68.5%) | 3271/4741 (69.0%) | 0.91 |
| Beta blocker | 3023/4851 (62.3%) | 67/111 (60.4%) | 2956/4740 (62.4%) | 0.67 |
| Statin | 4016/4854 (82.7%) | 94/111 (84.7%) | 3922/4743 (82.7%) | 0.58 |
Medication at time of hospital discharge is listed in Table 1 .
Most patients in the ALKK CAS registry were on dual antiplatelet therapy at the time of hospital discharge. Aspirin was documented in 95.7% and clopidogrel in 96.7% of all patients in the registry at time of hospital discharge. Vitamin K-antagonists were used in 5.5% of patients in the total population. A total of 82.7% of all patients treated by CAS were discharged on statins.
The in-hospital outcome of all patients in the registry is listed in Table 2 . The combined end point of death or stroke occurred in 3.1% (186 of 5,976) of all patients during their in-hospital stay. The in-hospital death rate was 0.5% (32 of 5,976) and the rate for ipsilateral stroke was 2.3% (134 of 5,947) in the total population. A contralateral ischemic event occurred in 0.9% (56 of 5,947) of all patients. Death or stroke rate was 4.0% (113 of 2,817) in symptomatic carotid stenoses compared with 2.2% (61 of 2,781) in asymptomatic stenoses (OR = 1.86, 95% confidence interval [CI] 1.36 to 2.56, p <0.001; Table 3 ).
Patients | Total (n=5976) |
|---|---|
| In-hospital events | |
| In-hospital death | 32/5976 (0.5%) |
| Intracranial bleeding | 13/3091 (0.4%) |
| Major stroke | 87/5947 (1.5%) |
| Minor stroke | 77/5947 (1.3%) |
| Transient ischemic attack (TIA) | 167/5947 (2.8%) |
| Amaurosis fugax | 23/5946 (0.4%) |
| Myocardial infarction | 3/5943 (0.1%) |
| Clinical events at 30-day follow up | |
| Death or stroke | 209/3943 (5.3%) |
| Death | 52/4217 (1.2%) |
| Intracranial bleeding | 14/2358 (0.6%) |
| Major stroke | 90/3875 (2.3%) |
| Minor stroke | 79/3859 (2.0%) |
| Transient ischemic attack (TIA) | 170/3893 (4.4%) |
| Amaurosis fugax | 26/3831 (0.7%) |
| Myocardial infarction | 7/3695 (0.2%) |
| Patient group | Death or stroke | Death | Non-fatal stroke |
|---|---|---|---|
| Total | 3.1% (186/5976) | 0.5% (32/5976) | 2.6% (154/5976) |
| Symptomatic patients | 4.0% (113/2817) | 0.7% (20/2817) | 3.3% (93/2817) |
| Asymptomatic patients | 2.2% (61/2781) | 0.4% (11/2781) | 1.8% (50/2781) |
| Embolic protection device | 2.6% (121/4713) | 0.5% (23/4713) | 2.1% (98/4713) |
| No embolic protection device | 5.5% (55/995) | 0.4% (4/995) | 5.1% (51/995) |
A logistic regression model, adjusting for possible confounding parameters, showed the following variables during CAS to be independent predictors of the combined end point of in-hospital death or stroke ( Figure 2 ): heart failure (OR 2.03, 95% CI 1.22 to 3.36, p = 0.006), symptomatic stenosis (OR 1.52, 95% CI 1.05 to 2.18, p = 0.03), and age (OR per 10 years 1.46, 95% CI 1.17 to 1.81, p <0.001). The presence of a visible thrombus at the lesion (OR 1.74, 95% CI 0.97 to 3.14, p = 0.06) and a length of the lesion >10 mm (OR 1.39, 95% CI 0.97 to 1.99, p = 0.07) also showed a trend toward an increased in-hospital death or stroke rate, but did not reach statistical significance. The use of an EPD was an independent protective variable against death or stroke (OR 0.42, 95% CI 0.25 to 0.73, p = 0.002).