Fibrothorax and Decortication of the Lung
Thomas W. Rice
Decortication of the lung is the process of peeling or stripping a constricting membrane from the pleural surfaces. Fowler17 and Delorme11 were the first physicians to describe freeing an entrapped lung, and in 1896 Delorme first used the term decortication.12 Decortication departed from the classic approach of aspiration, open drainage, and thoracoplasty for the treatment of chronic empyema and fibrothorax. Further refinements of the initial procedure included intercostal incision, wide exploration of the pleural cavity, full mobilization of the lung, removal of the fibrous peel (but not the visceral pleura), and suction drainage of the complicated pleural space.15,28,30,31,35,63
Despite individual surgical successes with decortication for chronic empyema, scant enthusiasm developed for this procedure. Milfeld and colleagues37 opined that this was due primarily to inadequacies of anesthesia, antimicrobial agents, blood transfusion, and technical expertise. Lack of therapy for organizing hemothorax complicating penetrating thoracic trauma reactivated interest in decortication during World War II.5,45,52,53,62 This experience, coupled with the development of effective antituberculous medication, encouraged many surgeons to explore decortication in the treatment of fibro- thorax and iatrogenic pneumothorax complicating pulmonary tuberculosis.1,19,20,21,40,41,64,65,66 More than 50 years after its inception, decortication of the lung in the treatment of fibrothorax was finally established. This surgical evolution was the result of one world war and three disease processes.
Today, an established fibrothorax is optimally managed by thoracotomy and decortication of the lung. Timely evacuation of hemothorax, empyema, or large pleural effusions with expansion of the underlying lung is critical in the prevention of fibrothorax. Tube thoracostomy, according to Huang and coworkers,22 is unlikely to successfully treat parapneumonic effusions if they are loculated or have a leukocyte count of no more than 6,400/μL. Failure of thoracentesis or tube thoracostomy should prompt early thoracoscopy or video-assisted thoracic surgery (VATS) drainage of pleural collections. In patients with empyema and hemothorax, videothoracoscopy and VATS facilitate early irrigation, debridement, and drainage of the pleural space.2,10,24,27,47,55,58 Despite earlier diagnosis and minimally invasive abilities to drain the pleural space, entrapment of the lung still occurs.59 Once organization has occurred, thoracotomy and decortication remain the treatment of choice.8,61
Pathophysiology
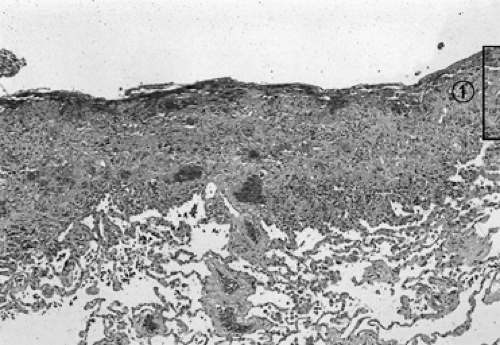
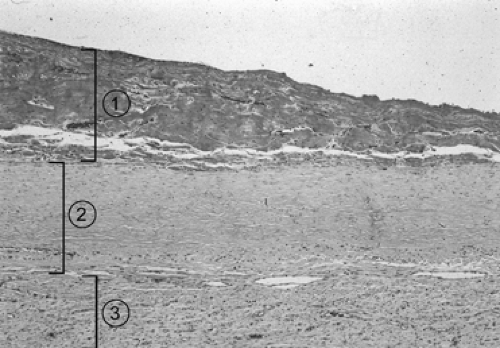
Samson51 pointed out that if pleural fluid is left undrained, regardless of its nature (Table 64-1), an inflammatory response deposits fibrin on the visceral and parietal pleura. As inflammation proceeds, a thin layer of immature blood vessels and loose collagen forms over the pleural surfaces (Fig. 64-1). Organization produces a dense, avascular collagen matrix that walls off the insulting fluid but does not affect the underlying pleura. In this fibrous cavity between the visceral and parietal pleura, the fluid decomposes (Fig. 64-2). The extent of inflammatory reaction is determined by the nature of the fluid and varies from a microscopically thin, glistening membrane in transudative pleural effusions to a thick, fibrous peel of empyema and hemothorax.
Fluid in the pleural space also results in pulmonary compression and, if sufficient in quantity, atelectasis. The compressed atelectatic lung is trapped by inflammatory tissue coating the visceral pleura. The underlying lung is usually unaffected unless the production of pleural fluid began as a destructive parenchymal process (e.g., tuberculosis, necrotizing pneumonia, or penetrating trauma). The chest wall and diaphragm are similarly coated by the fibrous process, which is typically thicker and more exuberant over the parietal pleura than over the visceral pleura. Entrapment of the lung and encasement of the thoracic cage produces a restrictive ventilatory defect and is characterized by reduction of lung volumes [forced vital capacity (FVC), total lung capacity (TLC), vital capacity (VC), forced expiratory volume in 1 second (FEV1), FEV50, FEV25], decreased diffusing capacity of the lung for carbon monoxide (DLCO) and carbon monoxide transfer coefficient (KCO), and an increase of the residual volume (RV)/TLC ratio.29 The DLCO corrected for these reduced volumes is normal, indicative of extrapulmonary restriction with a normal underlying lung. The alterations of pulmonary function in tuberculous pleural disease generally exceed those of tuberculous parenchymal disease.3
Pulmonary vasoconstriction results in a significant reduction of perfusion of the entrapped lung. Perfusion abnormality usually exceeds reduction of ventilation of the involved lung. This mechanism prevents hypoxia if contralateral lung function is preserved. Muller39 found that there is generally normal oxygen saturation at rest but moderate reduction with exercise.
In severe or bilateral disease, hypoxia, hypercarbia, pulmonary hypertension, and cor pulmonale may result.48
In severe or bilateral disease, hypoxia, hypercarbia, pulmonary hypertension, and cor pulmonale may result.48
Table 64-1 Causes of Fibrothorax | |
|---|---|
|
Diagnosis and Evaluation
Although a history of penetrating thoracic trauma, pneumonia, or tuberculosis may be elicited, no inciting cause will be found in 50% of patients.13 The most common complaint is progressive dyspnea on exertion. Occasionally patients may experience chest discomfort ranging from tightness to frank pain; dry, nonproductive cough; fatigue; or malaise. Ghoshal and colleagues18 have emphasized that cough with expectoration, hemoptysis, or fever should alert the examining physician to underlying pulmonary parenchymal disease. Physical examination will demonstrate unilateral fixation of the chest wall with reduced excursion of the diaphragm. The chest will be dull to percussion with impaired transmission of breath sounds on auscultation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree