INTRODUCTION
Congenital heart disease (CHD) is the most common group of congenital anomalies, occurring in 0.3–0.6% of live births but with a higher prenatal incidence. Fetal cardiology and the echocardiographic assessment of the fetal heart have developed exponentially over the past 30 years, and are now an established part of CHD practice internationally. There are large reported series describing virtually all forms of CHD and this expertise has led to a high degree of diagnostic accuracy, particularly in specialist centers. Technical advances mean the fetal heart can be examined transabdominally or transvaginally as early as the 1st trimester, particularly in fetuses at higher risk of developing CHD. Image acquisition by standard modalities such as 2D echocardiography, M-mode, pulsed-wave Doppler imaging and color flow Doppler mapping have become a routine part of fetal cardiac assessment. The advent of newer imaging modalities in the form of 3D echocardiography, tissue Doppler, and speckle tracking (myocardial deformation assessment) show promise in providing even greater insight into the structure and function of the fetal heart. With the improvements in the ability to diagnose congenital heart defects during fetal life, new challenges have emerged. These include risk stratification within diagnostic categories, fetal intervention, and optimization of postnatal management.
INDICATIONS FOR FETAL ECHOCARDIOGRAPHY
Traditionally, pregnancies have been classified as “low-risk” or “high-risk” based on whether there are specific fetal or historic risk factors for congenital heart disease. For “low-risk” pregnancies, ultrasound imaging of the fetal heart is typically incorporated into obstetric anomaly scans in the second trimester, which include views of the fetal heart. A variety of standards for imaging of the fetal heart have been published by different obstetric and cardiac professional bodies. Views of the heart which are common to all recommendations include the cardiac situs, four-chamber view, aortic valve/left ventricular outflow tract and pulmonary valve/right ventricular outflow tract, and/or three-vessel view. In pregnancies which are classified as “high risk,” the risk of congenital heart disease is elevated to a degree in which detailed fetal echocardiography is warranted. Detailed fetal echocardiography involves a comprehensive evaluation of the cardiac connections and cardiac function for which reference standards have been published. In specialist centers, detailed fetal echocardiography has a very high level of diagnostic accuracy for congenital heart defects. Reassurance of normality is also an important facet of evaluation of high-risk pregnancies. Some of the major indications for detailed fetal echocardiography are shown in Table 32.1.
First trimester assessment is used to identify fetuses at high risk of CHD of which measurement of nuchal translucency (NT) thickness is an essential component. The addition of ductus venosus flow patterns, particularly “a” wave reversal, and the presence of tricuspid valve regurgitation, has further refined this screening. NT screening was introduced to identify fetuses at increased risk for chromosomal abnormalities; however, there is a clear link with congenital heart disease. The relationship between NT and CHD is independent of the fetal karyotype, and the risk of CHD increases with the value of NT. There has been some debate as to the threshold NT which should warrant detailed fetal echocardiography. Most commonly either the 95th (2.6–3.2mm) or 99th percentiles (3.5mm) are used as “cutoff” values. Large series have suggested that NT screening may identify up to 30% of fetuses with CHD.
Newer, noninvasive screening techniques for trisomy 21, 13, and 18 are now available in the form of maternal blood cell–free (cf) DNA. This test does not exclude rarer chromosomal abnormalities or congenital heart defects. Current guidelines advise that it should be an adjunct to routine screening blood tests, and ultrasound scans.
TIMING OF FETAL ECHOCARDIOGRAPHY
Detailed images of the fetal heart can be obtained by transabdominal or transvaginal echocardiography as early as the 1st trimester and is particularly indicated in selected cases, for example, a previous child with severe cardiac disease, increased NT, or another structural fetal abnormality. This can provide early diagnosis or reassurance well in advance of the standard midtrimester anomaly scans. Most fetal cardiologists would recommend follow-up scanning even if an early scan, undertaken between 13 and 17 weeks, proves normal. The midtrimester period between 18 and 22 weeks gestation remains the optimal time period for fetal echocardiography and in many cases only a single scan will be required. Images obtained in the third trimester may not be as clear as second trimester images due to a reduction in available acoustic windows.
SYSTEMATIC APPROACH
For the purposes of screening many groups have advocated assessment of the fetal heart by a series of transverse or near transverse projections across the fetal thorax. A structured and systematic approach should be undertaken to ensure all aspects of the cardiac anatomy are examined (Table 32.2).
Fetal Position
Orientation of Fetal Position and Cardiac Situs
Before embarking on ultrasound assessment of the fetal heart it is essential to delineate the fetal position in the maternal abdomen (e.g., breech, cephalic, transverse) and the position of the fetal spine. This orientates the scan and establishes the anterior, posterior, left, and right of the fetus. This permits the operator to ensure that the heart and stomach are both on the left, and the position of the descending aorta and inferior vena cava to the left or right can be established with certainty. Normal abdominal situs is shown in Figure 32.1 (Video 32.1, Table 32.3). Normal cardiac situs (situs solitus) is determined by scanning in a plane immediately below the fetal heart and delineating the relationship of the descending aorta and inferior vena cava to each other and in relation to the fetal spine. In normal cardiac situs the abdominal aorta lies anterior and to the left of the spine. The inferior vena cava (IVC) lies anterior and to the right of the abdominal aorta and can be traced toward the morphological right atrium (RA). The fetal stomach should be seen on the left.
Four-Chamber View
The heart normally lies in the left of the chest, with the apex pointing to the left, at an angle of around 40°, filling one-third of the fetal thorax. In an optimal cross section, only a single rib should be visualized around the fetal thorax. It is essential to confirm the correct cut of the fetal thorax as “off-axis” images can be misleading or falsely reassuring. The stomach should be visualized to the left immediately below the heart. It is important to use the aforementioned method of orientation, as in certain situations the stomach and cardiac apex may lie on opposite sides or both may lie on the right of the fetus.
From the situs view, sweeping the probe cranially allows visualization of the four-chamber view of the heart. The normal four-chamber view of the fetal heart is shown in Figure 32.2 (Video 32.2, Table 32.4). This view allows assessment of the pulmonary venous drainage, atrioventricular (AV) connections, and the relative sizes of the atria and ventricles. The left ventricle (LV) is a smooth-walled chamber lying posteriorly. In comparison, the right ventricle (RV) is more trabeculated with a visible moderator band. Both ventricles should appear of similar size in a normal four-chamber view. The AV valves insert into the septum to produce the crux of the heart. The tricuspid valve inserts into the septum more apically and the mitral valve more toward the base, which is known as “differential insertion” (DI) or “AV valve offset.” This subtle anatomic feature is an essential component of the cardiac assessment. The offset is reversed where there are discordant atrioventricular connections, and loss of offsetting is a feature of atrioventricular septal (canal) defects (AVSD). A left superior vena cava (SVC) draining to a dilated coronary sinus can also make interpretation of offsetting more difficult. Color flow Doppler is used to assess flow patterns across the atrioventricular valves and to detect AV valve regurgitation. Color flow Doppler should also be used to interrogate the ventricular septum to identify ventricular septal defects which may not be seen on 2D imaging.

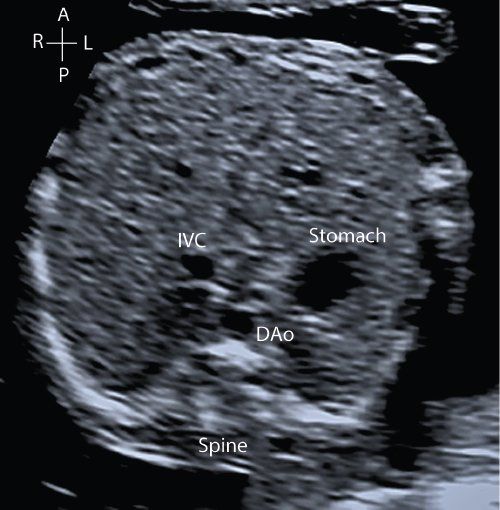
Figure 32.1. Transverse section through the fetal thorax demonstrating normal cardiac situs. The spine is seen posteriorly, with the stomach on the left. The descending aorta (DAo) lies anteriorly and to the left of the spine. The inferior vena cava (IVC) anterior and to the right of the DAo.
The mitral and tricuspid valves should be seen to open freely on 2D imaging and the addition of color flow Doppler will ascertain forward flow and the presence of any AV valve regurgitation. Trivial tricuspid valve regurgitation can be an incidental finding in the presence of otherwise normal cardiac anatomy and normal karyotype. The AV valve inflow pattern (diastolic filling) is assessed by placing the PW Doppler at the AV valve tips (Fig. 32.3). The fetus shows reversal of the E/A ratio and with advancing gestational age the E wave velocity increases so that there is also a gestation-related increase in the E/A ratio.
The connection of the pulmonary veins to the left atrium can be demonstrated on 2D imaging, but the addition of color flow Doppler assists in the confirmation that the veins drain directly into the left atrial mass. The velocity of blood flow through the pulmonary veins is lower than other cardiac structures and the color scale should be altered accordingly (Fig. 32.4, Video 32.3). In fetal life the foramen ovale is patent and can often appear aneurysmal. The flap valve of the foramen ovale is seen bowing into the left atrium, consistent with right-to-left shunting of blood at the atrial level, which can be confirmed by color flow Doppler. Some cardiac abnormalities are characterized by an abnormal four-chamber view of the heart. Examples include hypoplastic left heart (Fig. 32.5A, Video 32.4A(1–3)), tricuspid atresia (Fig. 32.5B, Video 32.4B), atrioventricular septal defect (Fig. 32.5C, Video 32.4C(1–2)) and discordance of the atrioventricular connections (Fig. 32.5D, Video 32.4D(1–2)). The abnormal features are outlined in the figure legend.
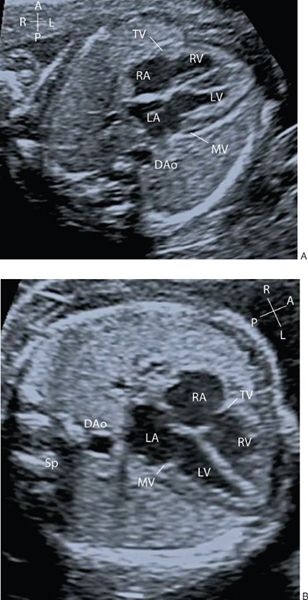
Figure 32.2. A: Transverse section through the fetal thorax demonstrating the four-chamber view. The left and right atria (LA, RA) and left and right ventricle (LV, RV) with corresponding mitral and tricuspid valves (MV, TV). B: Alternate four-chamber view demonstrating the AV valve offsetting.
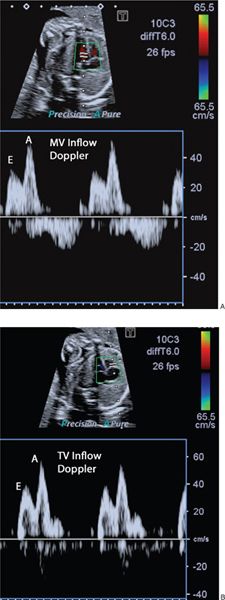
Figure 32.3. A: E and A wave of the mitral inflow B: E and A wave of the tricuspid inflow. During fetal life the E wave velocity is less than the A wave. The E/A ratio increases with advancing gestational age.
Echocardiographic Imaging of the Great Arteries
Remaining in the horizontal section and sweeping the probe cranially from the four-chamber view, the ventricular-arterial (VA) connections are visualized. The first outflow tract which is seen on sweeping cranially is the aorta and the second great artery is the pulmonary artery. The great arteries have a markedly different orientation within the fetal thorax. The left ventricular outflow tract (LVOT) and aortic valve arise from the center of the heart with the ventricular septum in continuity with the anterior wall of the aorta and the anterior leaflet of the mitral valve in continuity with the posterior wall of the aorta (Fig. 32.6A, Video 32.5). They course cranially toward the right shoulder before curving posteriorly and leftwards to form the transverse aortic arch. Color flow Doppler and pulsed-wave Doppler are used to assess both the direction and velocity of blood flow through the aortic valve (Figs. 32.6B and 32.7A; see Video 32.5). It is useful when assessing the Doppler velocity to also assess the fetal heart rate by measuring the time interval between each systolic contraction (Fig. 32.7B).
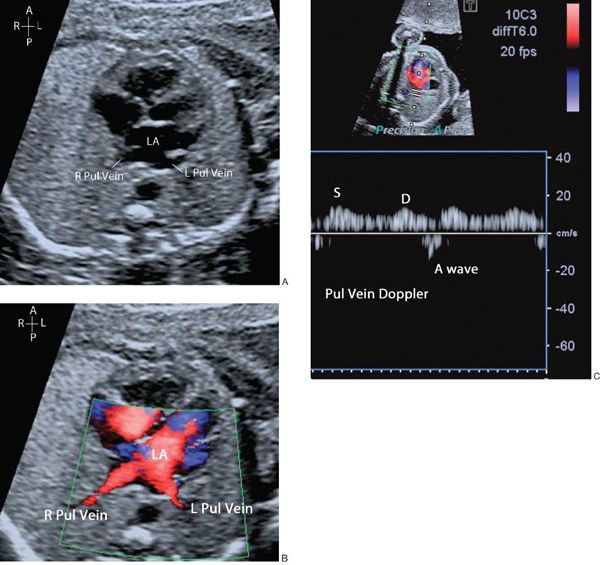
Figure 32.4. A: The right and left pulmonary veins (R, L Pul. veins) are seen to be confluent with the left atrium (LA). B: Antegrade blood flow demonstrated from the pulmonary veins into the LA. C: Normal pulmonary vein Doppler, with S, D, and A wave patterns.
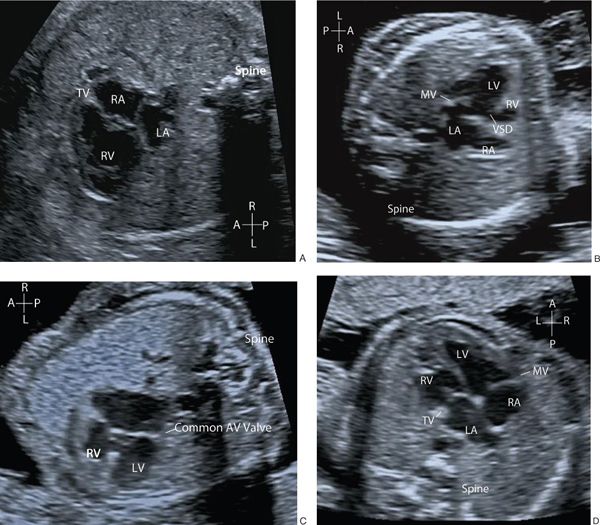
Figure 32.5. A: Hypoplastic left heart (HLH). The mitral valve is atretic with no discernible left ventricle. B: Tricuspid atresia (TAT) with a small right ventricle (RV) and ventricular septal defect. C: Atrioventricular septal defect (AVSD) with a common AV valve and associated ventricular and atrial septal defects. Note that there is no differential insertion of the atrioventricular valves and no visible “primum” atrial septum. D: Discordant atrioventricular connection. There is reversal of the normal pattern of offsetting of the atrioventricular valves. The left atrium connects to the morphologic right ventricle through the tricuspid valve which is more apically inserted than the mitral valve. The morphologic right ventricle is posterior to the morphologic left ventricle. The right atrium connects to the morphologic left ventricle via the mitral valve and the left ventricle is anterior.
Continuing to sweep cranially from the LVOT view allows assessment of the right ventricular outflow tract (RVOT) and pulmonary valve. The RVOT, pulmonary valve, and main pulmonary artery (MPA) arise anteriorly from the right ventricular mass and course straight back toward the spine, crossing anterior to the LVOT. (Fig. 32.8A, Video 32.6). It is important to note that in the normal heart the great arteries cannot be seen in the same plane and do not arise in parallel. This would raise the suspicion of transposition of the great arteries. As with the aortic valve, the addition of color flow Doppler will delineate direction of flow through the pulmonary valve and the velocity of blood flow measured with PW Doppler (Fig. 32.8B). Gestationally appropriate normative values are available for both aortic and pulmonary valve Doppler velocities. Sweeping cranially, the arterial duct is a continuation of the MPA into the descending aorta and is seen to travel directly back toward the left of the spine, dividing proximally into the left and right branch pulmonary arteries (PA) (Fig. 32.9, Video 32.7). In this projection, both PAs are not usually seen in the same plane as the arterial duct. More commonly, the duct and the right PA are seen together at a 45° angle and the left PA may be visualized in a longitudinal projection.
If the plane of insonation continues cranially in a near transverse plane cut through the superior mediastinum it will delineate the relationship, from left to right, of the MPA, aorta, and SVC. This is termed the three-vessel view (3VV). The relative sizes of the vessels seen in this projection is normally MPA > aorta > SVC, but the differences in size are usually quite small (Fig. 32.10A). This 3VV will also identify a left SVC or bilateral SVCs, which can be associated with other structural cardiac or extracardiac abnormalities, but may also be a variant of normal (Fig. 32.10B, Video 32.8, Tables 32.5 and 32.6).
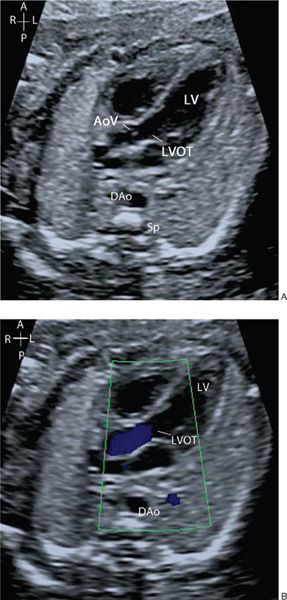
Figure 32.6. A: The left ventricular outflow tract (LVOT) is seen arising from the left ventricle (LV), with the aortic valve (AoV) in continuity with the mitral valve. B: Color flow Doppler demonstrates antegrade flow through the LVOT and aortic valve.
In a slightly more superior plane, the arterial duct and transverse aortic arch are seen to course back toward the left of the spine and meet in a “V” shape to become the descending aorta. The trachea lies to the right of the “V”—this view is termed the three-vessel tracheal view (Fig. 32.11, Video 32.9). In a right-sided aortic arch the transverse arch courses to the right of the trachea, and if the duct is left-sided these structures meet in a “U” shape with the trachea lying between the aorta and arterial duct. (Fig. 32.12, Video 32.10, Table 32.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


