Fig. 5.1
Histological representation of the alveolar region of a lung from (a; ×25 magnification) a term infant, and (b; ×50 magnification) a surfactant -treated preterm infant with BPD. Photomicrographs of a preterm infant with “healed” BPD, demonstrating (c; ×30 magnification) diffuse alveolar septal fibrosis (bottom) with enlarged alveoli (top), and (d; ×100 magnification) hyperplastic smooth muscle (arrow) surrounding a bronchiole (modified from [196])
Although BPD is a multifactorial disease, major factors in its etiology are thought to be inflammation , oxidative stress , and inappropriate repair mechanisms due to prolonged exposure to high oxygen concentrations (hyperoxia) during supplemental oxygen therapy or mechanical ventilation [89–91]. Mechanical ventilation can contribute to lung injury by inducing inflammation as a result of repetitive tissue stretch (shear stress) and over-inflation (barotrauma) of incompliant lung tissue; particularly damaging is repetitive opening of collapsed alveoli in atelectatic regions of the surfactant deficient lung [92]. Numerous other fetal and neonatal factors associated with preterm birth can contribute to the pathogenesis of BPD or alterations in lung development; such factors include the severity of prematurity, exposure to antenatal or postnatal corticosteroids, prenatal infection or inflammation, postnatal infection, intrauterine growth restriction, and inadequate postnatal nutrition (see Fig. 5.2) [91, 93]. Regardless of etiology, it is now apparent that persistent changes within the lungs of preterm infants, especially those who developed BPD, lead to an increased risk of poor lung function in childhood and late adolescence, as indicated by reductions in FEV1, forced mid-expiratory flow (FEF25–75 %), peak expiratory flow (PEF), transfer across the lung of carbon monoxide (TLCO), and increased airway resistance [94–99]. Individuals who were born preterm also have an increased risk of reduced exercise capacity [97, 98], asthma [94, 99–101], COPD [102], and respiratory infection s [103–105] later in life. The contribution of hyperoxia, mechanical ventilation, and corticosteroids to altered lung development, and later lung disease following preterm birth will be discussed in more detail in the following sections.
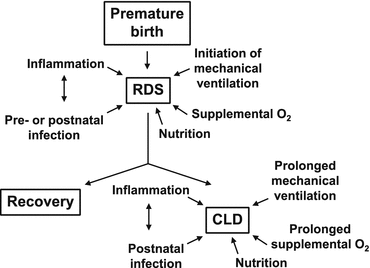

Fig. 5.2
Factors that are likely to contribute to respiratory distress syndrome (RDS) in infants who are born preterm, and factors that may lead to the subsequent development of chronic lung disease (CLD) [93]
Hyperoxia
Preterm infants are often administered hyperoxic gas to increase blood oxygen levels. Oxygen is added to inhaled gas to reach a target arterial oxygen saturation (SaO2) of 85–99 %, with the aim of preventing tissue hypoxia. In the past, the use of high oxygen concentrations (up to 100 % O2) was common, although concentrations were reduced once it became apparent that high concentrations could lead to tissue injury [106–108]. Currently, preterm babies are normally resuscitated using room air (21 % O2) and then the fraction of inspired oxygen (FiO2) is increased until adequate blood oxygenation levels are achieved [109]. However, as fetuses normally have an arterial partial pressure of oxygen (PaO2) of approximately 25–30 mmHg and a SaO2 of 70–80 %, exposure of an immature lung with an immature antioxidant system to even low supplemental oxygen concentrations may be detrimental. It is difficult to determine the contribution of neonatal hyperoxia alone to the long-term pulmonary outcomes of preterm birth and BPD. However, oxygen supplementation during the neonatal period in infants born very preterm has been identified as an independent risk factor for asthma in childhood [101]; further, prolonged oxygen therapy is associated with low FEV1 and the use of inhaled bronchodilators in very-low-birth-weight (preterm) children [96], suggesting an impact on the conducting airways .
Numerous experimental studies have shown that neonatal inhalation of hyperoxic gas can persistently alter the development of alveoli , conducting airways and pulmonary vasculature. Specifically, neonatal hyperoxia leads to fewer and larger alveoli (reviewed in [110]), altered alveolar epithelial cell differentiation [111], altered cellular composition of the bronchiolar epithelium [112–114], altered surfactant composition [111, 115], increased airway smooth muscle [116–119], decreased bronchiolar collagen [112], decreased pulmonary capillary density [120], and remodelling of the lung extracellular matrix [111, 121] and small pulmonary arteries [120]. Consequently, neonatal hyperoxia is associated with later reductions in lung function , including altered airway resistance and reactivity, dynamic lung compliance, tissue elastance and tissue damping, and pulmonary hypertension, as evidenced by right ventricular hypertrophy [111, 112, 120, 122]. Importantly, some of the effects of neonatal hyperoxia on the lung are long-lasting, with alterations in lung structure and function observed in early and advanced adulthood [111, 112, 118, 122–125]. Interestingly, recent studies and reviews have indicated that the oxygen concentration used and the duration of oxygen exposure are important determinants of lung injury [110, 111, 119].
Lung injury and alterations to lung structure that are induced by neonatal exposure to hyperoxia are thought to be due to oxidative stress and a subsequent pro-inflammatory response (see Fig. 5.3) (reviewed in [110]). Experimentally, neonatal treatments aimed at minimizing ROS and exploiting antioxidant defense, as well as treatments that target inflammatory cell influx into the lung and reduce the degree of lung inflammation , have shown promise in improving lung structure following neonatal hyperoxia [110]. Due to the importance of pulmonary vascular development in regulating alveolarization , treatments promoting angiogenesis following neonatal hyperoxia have also received much attention [110]. Recently, the use of stem/progenitor cells to repair or prevent lung injury has also been trialled in experimental models, and clinical trials for their use in preterm infants are currently underway, as discussed below.
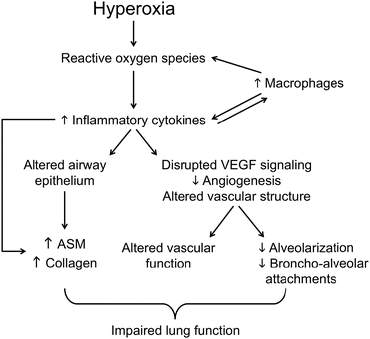

Fig. 5.3
Potential pathways whereby exposure of the developing lung to hyperoxia leads to alterations in the structure and function of the airways, pulmonary vasculature and alveoli , thus contributing to impaired lung function later in life. ASM airway smooth muscle , VEGF vascular endothelial growth factor [110]
Mechanical Ventilation
Owing to lung immaturity, preterm infants usually require assisted ventilation. Different ventilation strategies are employed depending on the requirement of the infant; these range from noninvasive strategies, including those involving constant airway pressure (i.e., CPAP using a constant or variable flow, or nasal cannula with low or high flow) or variable airway pressure (i.e., nIPPV, nasal positive airway pressure or nasal high-frequency ventilation), to invasive positive pressure ventilation requiring endotracheal intubation, including patient-triggered ventilation, volume target ventilation, and high-frequency oscillation [126]. It is now recognized that invasive ventilation strategies can increase the incidence of BPD and lung injury, which can increase the risk for persistent lung disease in infants born preterm. Furthermore, intubation is associated with trauma at the site of intubation (i.e., larynx and upper trachea ), as well as bacterial colonization, and sepsis [126]. Noninvasive ventilation strategies have been shown to reduce these adverse effects associated with mechanical ventilation, improve gas exchange, and reduce the incidence of BPD [127]. Thus, not only are noninvasive ventilation strategies now used for weaning infants off mechanical ventilation to reduce extubation failure, they are increasingly being used as primary methods of respiratory support for preterm infants with respiratory insufficiency. However, even noninvasive techniques have been associated with adverse effects, including pneumothorax, pulmonary over-inflation, increased work of breathing, decreased cardiac output, as well as nasal complications and gastrointestinal distension and perforation [126]. Large randomized controlled trials are required to determine the beneficial effects of different modes of noninvasive ventilation on long-term respiratory and non-respiratory outcomes.
Ventilator-induced lung injury is believed to be a result of either volutrauma or barotrauma, which is injury related to over-expansion of the lungs (due to high volumes or pressures), atelectrauma, which is injury caused by repeated opening of collapsed alveoli , or biotrauma, which is injury caused by up-regulation of pro-inflammatory responses to mechanical ventilation, or a combination of these factors [126]. In the clinical setting, it is difficult to determine the precise cause of lung injury in preterm infants due to the multifactorial and individualized nature of their care, both before and after birth. Experimentally, it has been shown in rodents and sheep that even brief mechanical ventilation (2–24 h) of the developing lung alone (i.e., without other confounding factors) leads to impaired formation of alveoli and lung microvessels, as indicated by decreases in secondary crest density, alveolar number, and CD31 staining, and increases in airspace and alveolar size; these changes in lung structure are likely due to disruption of elastin deposition in the lung [92, 128–130] and altered expression of other genes/proteins that regulate alveolar formation and angiogenesis, including platelet derived growth factor–A (PDGF–A), tenascin–C, and vascular endothelial growth factor receptor 2 (VEGF-R2) (see Fig. 5.4) [92, 131]. Studies have also identified numerous transcription factors and genes that are dysregulated by mechanical ventilation, contributing to lung injury and altered lung development ; likely factors include those involved in TGF-β signalling, of which elastin is a target, mechanotransduction and the immune response [132–134]. Furthermore, recent studies have shown that invasive mechanical ventilation in preterm lambs causes an increase in histone deacetylatase (HDAC) 1 and subsequent genome-wide histone hypoacetylation in the lung, indicating alterations in the epigenetic regulation of gene expression [135]. Insulin-like growth factor-1 (IGF-1) is one gene whose expression is increased in mechanically ventilated preterm lambs and in infants who have died from RDS or BPD, and which appears to be epigenetically modified by mechanical ventilation [135]. Further studies are required to identify other genes that are modified by mechanical ventilation and the specific pathways leading to their alteration [93]. A greater understanding of the mechanisms by which mechanical ventilation alters gene expression and leads to altered lung development will enable targeted interventions to be developed. In this regard, histone acetylation is increased and alveolar formation is improved when preterm lambs that are mechanically ventilated are treated with HDAC inhibitors [135].
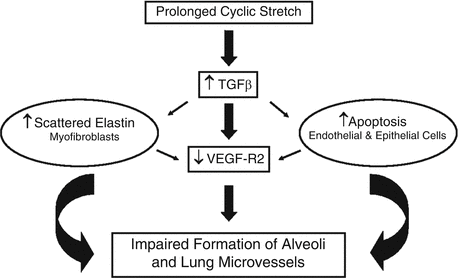

Fig. 5.4
Potential pathways whereby prolonged cyclic stretch of the lung caused by positive-pressure mechanical ventilation alters the formation of the pulmonary vasculature and alveoli . TGF–β transforming growth factor-β, VEGF–R2 vascular endothelial growth factor receptor 2 [92]
In addition to altering alveolar structure, a short period (2–12 h) of positive pressure ventilation of the immature (prenatal) sheep lung induces injury and remodelling of the small conducting airways (i.e., bronchioles), including epithelial cell detachment, alterations in the areas of epithelium and collagen in intact airways, and an increase in airway smooth muscle [129, 130, 136]. There is evidence that airway remodelling persists in the absence of further ventilation, with changes in epithelial area, epithelial cell proliferation and apoptosis, airway smooth muscle and collagen, and a decrease in the number of bronchiolar-alveolar attachments [129, 130, 136]. There is also evidence, however, that the fetal lung is capable of spontaneous repair following ventilation-induced injury, with normalization of alveolar structure in the absence of continued ventilation [129, 130], although this recovery could potentially be restricted to the fetal lung. Changes in alveolarization and airway wall structure following mechanical ventilation could contribute to the increased risk of poor lung function and obstructive lung disease reported in individuals who were born preterm. Indeed, airway reactivity is increased 48 h after brief mechanical ventilation of rat pups [137]. Further studies investigating the long-term effects of mechanical ventilation of the immature lung on respiratory health are needed.
Corticosteroids
Corticosteroids have been used in preterm infants with evolving or established BPD to reduce lung inflammation and improve gas exchange; corticosteroids are used to reduce the need for oxygen supplementation [91] and to decrease the risk of retinopathy of prematurity and death [138]. Postnatal corticosteroids, predominantly dexamethasone, are generally administered systemically and can be given early (≤7 days after birth) or late (>7 days). Although systemic corticosteroid therapy can have short-term benefits, it has been linked to adverse long-term outcomes, including detrimental effects on lung structure, neurodevelopment, cardiovascular function, and the gastrointestinal system [91, 138]. Although many of these effects have been linked to the prolonged use of high-dose corticosteroids (especially dexamethasone), studies have indicated that even low-dose corticosteroids may have adverse effects [91]. Inhaled corticosteroids have been trialled in preterm infants with the aim of reducing the side effects associated with systemic corticosteroid use, although these have had limited success [91, 138]. Studies investigating the optimal timing, dose, and type of corticosteroids given to preterm infants to improve short-term outcomes and reduce adverse long-term outcomes are warranted.
Nutrition
Adequate nutrition plays a key role in normal lung development . Experimentally, reduced nutrition during lung development before and after birth can affect alveolarization and bronchiolar structure [28, 31, 139]. The effects of undernutrition during fetal life on lung development have been described above. Lung development continues after birth and it has been shown in sheep that, at maturity, animals that experienced postnatal growth restriction have a smaller lung volume and a smaller pulmonary surface area, relative to lung and body size, than animals that grow normally [140]. Undernutrition during the early postnatal period has been shown to disrupt lung development, especially when combined with exposure to hyperoxia [112, 141, 142]. This is of particular importance in the context of very preterm infants who form most of their alveoli after birth. In addition, very preterm infants have a high risk of developing BPD, and it is known that malnutrition represents one of the many factors contributing to BPD [143]. Maintaining a careful balance of nutritional support for very and extremely preterm infants represents a considerable challenge. Interventions that are necessary to support the survival of these preterm infants, combined with developmental complications they may encounter (e.g., prolonged orotracheal intubation, medications that alter gastrointestinal motility, altered palatal groove formation, irritability due to neurological status, gastrointestinal immaturity) make feeding by mouth a problematic task; therefore, nutritional support is often provided via parenteral or enteral routes [144]. However, most extremely preterm infants receive minimal enteral feeding during the first two weeks of life, due to the abovementioned problems, and their nutritional requirements need to be supplemented with parenteral feeding. Recently, it was reported that a critical minimal amount of enteral feeding is required to prevent the development of BPD [145]. Preterm infants who developed BPD received less enteral feeding during the first two weeks of life, even though it was compensated by parenteral nutrition [145]. Furthermore, it has been reported that, compared to preterm infants without BPD, those with BPD have a greater energy expenditure (up to 25 % above total caloric needs), likely due to their increased work of breathing, stress, inflammation , medications, and the perceived need for catch-up growth [143, 144].
Another aspect of nutritional management of preterm infants that has been associated with an increased risk for BPD is fluid intake. It has been reported that high fluid intake during the first days after birth may increase the risk of developing BPD, through the persistence of a patent ductus arteriosus and a higher fluid content in the pulmonary interstitial tissue; these complications could contribute to decreased lung compliance and increase the need for respiratory support [143, 146]. Overall, it is suggested that adequate nutritional management of preterm infants with evolving or established BPD can be maintained by adopting enteral nutrition as soon as possible, avoiding excess fluid administration during the first days after birth, and progressively increasing energy intake [147].
The use of specific micronutrients, such as vitamins A, C, and E, inositol, iron, selenium, glutamine, cysteine, and methionine, may be beneficial in the nutritional management of preterm infants to prevent or lessen the severity of BPD (reviewed in [144]). Indeed, optimal feeding and growth in preterm infants remains a challenge, and overnutrition of the preterm infant to achieve catch-up growth may actually be detrimental to long-term health [148]. Although accelerated growth of the preterm infant has beneficial effects on neurodevelopment, it has also been associated with many metabolic and cardiovascular implications that present in childhood and adulthood [148]. Further research is needed in order to identify the optimal method of managing preterm nutrition with the goal of enhancing lung growth, preventing BPD, and avoiding long-term, potentially harmful programming effects of catch-up growth.
Although infants born preterm are faced with a vulnerable period during which nutrition can greatly affect their lung development and subsequent lung health, nutritional status can also impact upon the mature lung. Emphysema is commonly observed in patients with anorexia nervosa and was also noted at autopsies of humans who died from starvation [149]. These findings support the concept that lung structure/function is dependent upon nutritional status.
Respiratory Infections
The link between early-life respiratory infection s, particularly viral infections, and subsequent increased risk of recurrent wheezing and asthma is being increasingly investigated. Viral respiratory infections during infancy have been shown to contribute to the development of wheezing in childhood. In particular, the occurrence of symptomatic rhinovirus infection during infancy (<12 months of age) has been identified as the most significant risk factor for the development of wheezing 1 year after infection [150] and into early childhood [151]. Infection with respiratory syncytial virus (RSV) in infancy has also been linked with wheezing in pre-school children, albeit with a weaker relationship compared to rhinovirus infections [151]. A later study confirmed these findings, showing that bronchiolitis caused by viruses other than RSV was associated with a greater risk of recurrent wheezing (within the first 2 and 3 years after hospitalization for bronchiolitis) compared to RSV-associated wheezing [152]. Not only has rhinovirus infection been associated with recurrent wheezing, but rhinovirus infection during the first 3 years of life is also significantly associated with reduced lung function (lower FEV1, percent predicted FEV1, FEV0.5, FEF25–75 %, FEV1/forced vital capacity (FVC), and FEV0.5/FVC) at 8 years of age compared to children with non-wheezing rhinovirus illnesses and no rhinovirus illnesses [153]. Furthermore, this study showed that children with RSV-induced wheezing did not have significant alterations in any of the lung function measurements, indicating that infections in early childhood caused by rhinovirus, rather than RSV, are significant predictors of decreased lung function in childhood [153]. Not only are viral respiratory infections during early postnatal life associated with subsequent wheezing, but they often precede the development of childhood asthma. In a cohort of children that was followed up to 6 years of age after specific viral respiratory wheezing illnesses in early childhood (within the first 3 years of life), rhinovirus-associated wheezing illnesses were the most significant predictors of the subsequent development of asthma [154]. This, however, does not exclude RSV as a potential risk factor for asthma. Recent studies showed that severe RSV-induced bronchiolitis during infancy (<12 months of age) is associated with an increased risk of asthma at 6 and 7 years of age [155, 156]. Furthermore, RSV infection in infancy was shown to be associated with a 3.2-fold increase in the risk of wheezing and impaired lung function (lower FEV1, FVC, and FEV1/FVC, and increased respiratory system resistance) at age 6 [155]. Another recent study reported on a study population at 15–18 years of age, and compared patients hospitalized for bronchiolitis in infancy (<24 months of age) caused by RSV or rhinovirus to a control population [157]. This study showed that patients hospitalized in infancy for bronchiolitis had an increased risk of asthma in adolescence; of the bronchiolitis patients, those who had rhinovirus infection had a higher risk of self-reported asthma, but not doctor-diagnosed asthma, than those who had RSV infection [157]. Studies that have extended further into adulthood have found that hospitalization for bronchiolitis in infancy (<24 months of age) is associated with an increased risk of asthma, and an increased use of asthma medication at 28–31 years [158]. In that study, doctor-diagnosed asthma was present in adulthood in one-third of former bronchiolitis patients, whereas asthma was present in only 10 % of controls. An earlier study of the same cohort demonstrated that the prevalence of asthma at 28–31 years was similar to that recorded 10 years earlier at 18–21 years of age [159], indicating the early-onset and persistence of long-term sequelae following early-life respiratory infection. However, these studies did not differentiate between RSV and rhinovirus bronchiolitis. The link between early-life respiratory infections and subsequent long-term respiratory morbidity has also been highlighted in a follow-up study that showed an association between RSV bronchiolitis in the first year of life and increased prevalence of asthma or recurrent wheeze at 18 years of age; 39 % of RSV subjects vs. 9 % of control subjects [160]. A reduction in spirometric airway function was reported in the RSV cohort compared to controls, regardless of whether the RSV subjects presented with or without asthma or recurrent wheeze [160].
Bacterial colonization of the hypopharynx with S. pneumonia, H. influenza, or M. catarrhalis in healthy neonates at 4 weeks of age has been associated with an increased risk of pneumonia and bronchiolitis in early life (up to 3 years) independent of asthma [161]; this suggests that an altered microbiome of the neonatal airway may affect the maturation of the immune system, leading to an increased risk of pneumonia and bronchiolitis. Furthermore, radiologically confirmed pneumonia in the first 2 years of life has been associated with asthma or asthma-like symptoms in pre-school children (4–5 years of age) [162]. This is confirmed in a meta-analysis that showed an overall risk of major long-term respiratory sequelae following childhood pneumonia (before 5 years of age) in non-hospitalized children of 5.5 %; this risk was almost three times greater in children who had been hospitalized, with adenovirus pneumonia having the highest risk (approximately 55 %) [163]. Major respiratory sequelae were defined as restrictive lung disease, obstructive lung disease, and/or bronchiectasis, and the most common type of major outcome reported was restrictive lung disease [163]. These findings indicate that children diagnosed with pneumonia should be considered at risk of long-term respiratory sequelae, even after acute symptoms have resolved.
Environmental Exposures
Many studies have highlighted the deleterious effects of a suboptimal environment on lung development (reviewed in [164]). Harmful environmental exposures include both outdoor pollutants (e.g., industrial and motor vehicle emissions) and indoor pollutants (e.g., environmental tobacco smoke (ETS) and fungal spores). In particular, ETS exposure in early life has been identified as a significant risk factor for poor pulmonary outcome in later life. A recent meta-analysis indicated that passive exposure to ETS (in both pre- and postnatal life) increases the incidence of wheeze and asthma in children and adolescents by at least 20 % [165]. Maternal smoking was associated with a 52 % increase in the risk of wheeze and a 20 % increase in the risk of asthma at 5–18 years of age [165]. Not only has ETS exposure during infancy been associated with increased risk of wheeze and asthma, but a meta-analysis has also shown it to increase the risk of lower respiratory infection s, particularly bronchiolitis [166]. All types of passive ETS exposure (pre- and postnatal maternal, paternal, both parents, any household member smoking), but particularly maternal smoking, led to a significant increase in the risk of infants developing lower respiratory infections in the first 2 years of life [166]. This meta-analysis highlights the importance of limiting ETS exposure during both gestation and childhood to prevent the onset of wheezing, asthma, and respiratory infections. In contrast to the large number of reports on effects of ETS exposure, there are few reports on the effects of exposure to traffic-related air pollution during infancy and childhood and its effects on later respiratory morbidity. A recent systematic review and meta-analysis showed that early childhood exposure to traffic-related air pollution was associated with the development of asthma throughout childhood up to 12 years of age, with the magnitude of risk increasing with age [167]. However, further studies are needed to investigate the relation between effects of exposure at specific ages and adult outcomes.
Potential Targets for Cell-Based Therapies: Neonatal Treatments for Preterm Infants
The use of cell-based therapies to prevent or repair neonatal lung injury in very preterm infants has now evolved from experimental studies using animal models to phase I and II clinical trials in very preterm infants at risk of BPD. The rationale for the current clinical trials is based on knowledge gained from studies examining the perturbation of pulmonary stem cells in neonatal lung injury, as well as experimental studies in animal models that found stem cell-based therapies to be beneficial. Studies in animal models and in humans have shown that altered function or depletion of resident stem cells within the developing lung likely contributes to the pathogenesis of BPD. In rodent models of BPD, in which newborn pups are exposed to high concentrations of oxygen, lung epithelial cells, mesenchymal stromal cells (MSCs), and endothelial progenitor cells (EPCs) were shown to be significantly reduced in number and also displayed altered differentiation potential [123, 168–170]. Similar perturbations to resident stem cells have been reported in tracheal aspirates and umbilical cord blood samples taken from preterm infants [171–175]. The presence of MSCs in tracheal aspirates has been reported as an indicator of BPD development , and these MSCs were shown to demonstrate a lung-specific gene expression and to secrete pro-inflammatory cytokines; this suggests that the MSCs in the tracheal aspirates originated from the lungs and have the capacity to contribute to lung injury [171, 172]. In samples of umbilical cord blood from preterm infants, endothelial colony forming cells (ECFCs; a subset of EPCs) were shown to have an increased susceptibility to in vitro exposure to hyperoxic gas, and were found in reduced numbers among BPD infants compared to non-BPD preterm infants [173–175]. This suggests that a reduction in the levels of angiogenic progenitor cells may contribute to disrupted vasculogenic mechanisms during lung development and lead to impaired vascular and alveolar growth. In response to the findings of stem cell perturbation in BPD, experimental studies have focused on their therapeutic replacement with exogenous-derived stem cells (reviewed in [176]). One of the most commonly researched cell types is the MSC, which can be easily sourced from bone marrow, adipose tissue, muscle, peripheral blood, umbilical cord blood, umbilical cord Wharton’s jelly, and placenta. Numerous components of neonatal lung injury have been attenuated by the administration of MSCs, including inflammation , vascular damage, impaired alveolarization , and fibrosis [170, 177–188]. Furthermore, MSCs have been reported to have low engraftment and differentiation into the lung, suggesting a paracrine-mediated therapeutic effect. This has been supported by studies showing similar protective properties of cell-free conditioned media from MSCs in experimental models of neonatal lung injury. Other cell types (ECFCs, angiogenic cells, and amnion epithelial cells) have also been examined in experimental studies and have shown promise in their therapeutic benefit for neonatal lung injury [189–194].
The overall aim of experimental studies on neonatal lung regeneration has been to generate evidence to create a rationale for translating experimental findings into the clinic. Encouragingly, there has recently been translation of findings from animal studies into clinical trials in preterm infants, with the results of the first phase I clinical trial (NCT01297205) of stem cell therapy in preterm infants being published [195]. The authors assessed the safety and feasibility of human umbilical cord blood-derived MSCs, administered to nine very preterm infants who were at risk for developing BPD. The infants were born at 24–26 weeks of gestation (630–1030 g) and needed continuous ventilatory support that could not be decreased due to significant respiratory distress prior to enrolment into the study. Three patients were given a low dose of MSCs (1 × 107 cells/kg) via the trachea , and six were given a high dose (2 × 107 cells/kg). Treatment-related serious adverse effects were assessed for 84 days following transplantation of the MSCs. The authors reported no serious adverse effects or dose-limiting toxicity associated with transplantation of the MSCs. There was no significant difference in serious adverse effects between the low-dose and high-dose MSC group or between the MSC group and a matched-comparison group, except in BPD severity; the MSC group had significantly lower BPD severity than the matched-comparison group. Furthermore, the inflammatory markers matrix metalloproteinase (MMP)-9, interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, and TGF-β were reduced in tracheal aspirate fluid after MSC transplantation. The findings of this study are important in establishing the feasibility, safety, and efficacy of MSC treatment for BPD in preterm infants. Follow-up studies are currently underway to assess the long-term safety of MSC transplantation in the same preterm infants reported in the phase I clinical trial, at approximately 2 years of age (NCT01632475) and 5 years of age (NCT02023788). Furthermore, a randomized, double-blind, multicenter, phase II clinical trial (NCT0182957) has commenced that will investigate the efficacy and safety of a low dose of MSCs versus a control group for the treatment of BPD in preterm infants; this trial will be followed up with another study (NCT01897987) investigating the long-term safety and efficacy of the phase II trial. It is anticipated that findings from these current clinical trials will pave the way for the future treatment of neonatal lung diseases using stem cell-based therapies.
Conclusions
The lung develops during prenatal and early postnatal life, and is therefore vulnerable to adverse environmental conditions during the embryonic, fetal, and postnatal stages of lung development . The peripheral lung, including alveoli , the pulmonary microcirculation, and small conducting airways , is especially vulnerable to injury or altered development . Owing to the lung’s limited capacity for repair , lung injury, or altered lung development caused by a suboptimal fetal or early postnatal environment can persist into later life, adversely affecting adult lung function and increasing the risk of respiratory illness. Changes in lung structure can be due to a direct developmental effect or to changes in gene and protein expression caused by alterations in the nutritional, physical/mechanical, oxidative, or immunological environments. In addition, a range of environmental factors may induce epigenetic alterations in key genes that regulate lung development to cause persistent alterations in lung structure and/or function. Although there are currently no treatments capable of repairing the injured lung, there is now encouraging experimental evidence on the effectiveness of cell-based therapies to prevent or repair injury to the immature lungs, with clinical trials now underway in infants who are born very preterm and are at risk of developing chronic lung disease.
References
1.
Martinez FD (2009) The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc 6:272–277PubMedCentralPubMed
2.
Barker DJ, Thornburg KL (2013) Placental programming of chronic diseases, cancer and lifespan: a review. Placenta 34:841–845PubMed
3.
Vickers MH (2014) Early life nutrition, epigenetics and programming of later life disease. Nutrients 6:2165–2178PubMedCentralPubMed
4.
Narang I, Bush A (2012) Early origins of chronic obstructive pulmonary disease. Semin Fetal Neonatal Med 17:112–118PubMed
5.
Pallotto EK, Kilbride HW (2006) Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol 49:257–269PubMed
6.
Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO (1991) Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 303:671–675PubMedCentralPubMed
7.
Lawlor DA, Ebrahim S, Davey Smith G (2005) Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax 60:851–858PubMedCentralPubMed
8.
Cetin I, Alvino G (2009) Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 30:S77–S82PubMed
9.
Pecks U, Brieger M, Schiessl B, Bauerschlag DO, Piroth D, Bruno B et al (2012) Maternal and fetal cord blood lipids in intrauterine growth restriction. J Perinat Med 40:287–296PubMed
10.
Briana DD, Malamitsi-Puchner A (2013) Small for gestational age birth weight: impact on lung structure and function. Paediatr Respir Rev 14:256–262PubMed
11.
Dezateux C, Lum S, Hoo AF, Hawdon J, Costeloe K, Stocks J (2004) Low birth weight for gestation and airway function in infancy: exploring the fetal origins hypothesis. Thorax 59:60–66PubMedCentralPubMed
12.
Minior VK, Divon MY (1998) Fetal growth restriction at term: myth or reality? Obstet Gynecol 92:57–60PubMed
13.
Tyson JE, Kennedy K, Broyles S, Rosenfeld CR (1995) The small for gestational age infant: accelerated or delayed pulmonary maturation? Increased or decreased survival? Pediatrics 95:534–538PubMed
14.
Kotecha SJ, Watkins WJ, Heron J, Henderson J, Dunstan FD, Kotecha S (2010) Spirometric lung function in school-age children: effect of intrauterine growth retardation and catch-up growth. Am J Respir Crit Care Med 181:969–974PubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


