Chapter 18 Extracranial Cerebrovascular Disease
The Carotid Artery
Historical Review
The earliest report linking cervical carotid artery disease to stroke is credited to Savory,1 who in 1856 described a young woman with left monocular symptoms in combination with a right hemiplegia and dysesthesia. Postmortem examination demonstrated an occlusion of the cervical portion of the left internal carotid artery, along with bilateral subclavian artery occlusions. In 1875, Gowers reported a similar case,2 and subsequent reports of individual cases were made by Chiari in 1905,3 Guthrie and Mayou in 1908,4 and Cadwater in 1912.5 By 1914, Hunt,6 in an important publication, emphasized the relationship between extracranial carotid artery disease and stroke. He also described the phenomenon of intermittent cerebral symptoms associated with partial occlusion and used the term cerebral intermittent claudication as a characterizing analogy. Hunt also pointed out that the clinicopathologic observations in patients with stroke were hampered by the fact that routine autopsies did not include examination of the cervical carotid arteries (as is often the case today because of the desire to maintain access to the external carotid artery for the mortician). He emphasized that no examination of cerebral infarction can be considered complete without examination of the neck vessels.6
The next major step in the evolution of the management of extracranial cerebrovascular disease came with the development of carotid angiography by Moniz in 1927.7 By 1937, Moniz and colleagues had described four cases of internal carotid occlusion diagnosed by angiography.8 In 1938, Chao and colleagues added two more cases,9 and by 1951, Johnson and Walker had collected from the world literature a total of 101 cases of occlusion of the cervical carotid artery diagnosed by angiography.10 Despite these early observations, the medical world was still slow to appreciate the relationship between extracranial cerebrovascular disease and cerebral symptoms, as emphasized by the fact that when cerebral angiography came into common use for neurologic diagnosis in the 1950s and 1960s, only the intracranial vessels were included on radiographs films. The area of the carotid bifurcation was seldom examined. By the late 1950s, patients with hemiplegia were still commonly receiving diagnoses of a middle cerebral artery thrombosis, without consideration of the carotid bifurcation as a source of the problem.
The next major steps in the evolution of understanding came from reports by Fisher in 1951 and 1954.11,12 Fisher reemphasized the relationship between extracranial arterial occlusive disease and cerebral symptoms. He also pointed out that the lesion could be either total occlusion or stenosis. His most important observation, however, was that the disease was often localized to a short segment of the carotid artery, and he predicted that surgical correction might be possible if patients could be identified in the early stages of the clinical syndrome. Fisher stated, “It is even conceivable that some day vascular surgery will find a way to bypass the occluded portion of the artery during the period of ominous fleeting symptoms. Anastomosis of the external carotid artery or one of its branches with the internal carotid artery above the area of narrowing should be feasible.”
The surgical phase of understanding and managing extracranial cerebrovascular disease probably began in 1951, but it was not reported in the literature until 1955. This early report by Carrea and colleagues13 from Buenos Aires described their experience with the management of a patient with carotid artery stenosis. They resected the diseased internal carotid artery and performed an anastomosis between the external carotid artery and the distal internal carotid, as predicted earlier by Fisher.11 In 1953, Strully and coworkers14 attempted a thromboendarterectomy of a totally thrombosed internal carotid artery. This attempt was unsuccessful, but the authors suggested that thromboendarterectomy should be technically feasible before thrombosis occurred, as long as the internal carotid artery is patent distally. The first carotid endarterectomy was probably performed by DeBakey and colleagues in an operation done on August 7, 1953, but it was not actually written up until 1959 and then reviewed in 1975.15,16 The report that was most important in calling the world’s attention to the feasibility of carotid artery reconstruction came from Eastcott and associates.17 Their operation was performed on May 19, 1954, on a patient who was having hemispheric transient ischemic attacks (TIAs) with demonstrable disease at the carotid bifurcation; they used direct, end-to-end anastomosis between the common carotid artery and the internal carotid artery distal to the atherosclerotic lesion.
Although operations on the carotid artery were in the early phase of development, surgical attack was also considered feasible on occlusive lesions of the major arch vessels. In 1956, Davis and colleagues18 reported their experience with endarterectomy of the innominate artery performed on a patient on March 20, 1954. In 1957, Warren and Triedman reported the second case.19
Thompson,20 in his 1996 Willis lecture, related in great detail the history of surgery to prevent stroke. Those interested in the definitive history will be rewarded by reading this excellent paper.
Natural History of Extracranial Arterial Occlusive Disease
In the United States, approximately 600,000 people suffer a first stroke each year. In 200,000 of these cases, death follows, but at any one time approximately 1 million stroke victims are alive and disabled. In 1976, the annual direct and indirect cost of stroke was estimated at $7,363,784,000.21 Nearly 30 years later, with inflation and the accelerating cost of medical care, this cost has probably quadrupled. The incalculable morbidity of the affected individual adds further to the magnitude of this problem. Prevention remains the most plausible alternative.
The initial mortality of an ischemic stroke ranges from 15% to 33%.22–24 Survivors remain at an inordinately high risk of subsequent stroke, estimated between 4.8% and 20% per year.25,26 This implies that half of patients will experience a second event within 5 years.23,27,28 The average recurrent stroke rate reported in the literature is between 6% and 12% each year. The most common cause of death in patients with extracranial arterial occlusive disease is myocardial infarction (MI). In an analysis of 535 stroke victims, however, the leading cause of death was recurrent stroke, as opposed to the expected myocardial mortality.26
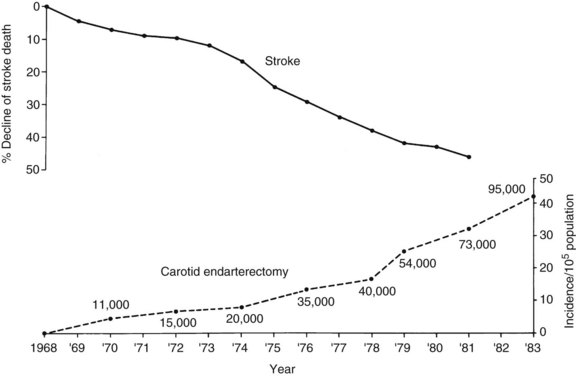
Since 1973, public health statistics have documented an accelerating decline in stroke mortality.29 Stroke used to be considered the third leading cause of death in the United States. The American Heart and Stroke Association recently reported that stroke has now dropped to the fourth leading cause of death. The reasons for this are multifactorial but include successful efforts at primary prevention including surgical intervention and treatment of carotid bifurcation lesions, improved medical management, and better care of the patient with acute stroke, often in specialized centers. This reduction in stroke mortality has led to the erroneous assumption that a decline has also occurred in stroke incidence, which is not the case.
In 1989, Wolf and colleagues30 reported the epidemiologic data from the Framingham Study to the 14th International Joint Conference on Stroke and Cerebral Circulation. They reviewed the experience from three successive decades, beginning in 1953. A decline in stroke fatality in both men and women was observed. However, the 10-year prevalence of stroke actually rose, and the incidence of stroke in men rose from 5.7% to 7.6% to 7.9%, without any apparent change in women. The authors postulated that falling case fatality rates might have resulted from changes in diagnostic criteria, a lessening in stroke severity, or improved care of stroke patients.
Harmsen and colleagues31 reviewed the stroke incidence and fatality in Gothenburg, Sweden, between 1971 and 1987. They noted that the stroke incidence remained the same during that interval, but the stroke fatality rate declined in both sexes. This was more marked for intracerebral hemorrhage and subarachnoid hemorrhage than for infarction. They concluded that the decline in stroke fatality rates might have been related to a decrease in smoking or better management of blood pressure. They could not explain why no corresponding decline in stroke incidence occurred.
Finally, Moden and Wagener32 examined the epidemiologic aspects of stroke based on death certificate information available from the National Center for Health Statistics’ compressed mortality file for all 50 states and the District of Columbia for the period 1968 to 1988. They noted a decline in stroke mortality that continued through the 1970s and 1980s, whereas morbidity remained constant and possibly even increased. They noted similar morbidity and mortality rates in both sexes. They concluded that the observed decrease in stroke mortality rates resulted from improved survival rather than a decline in incidence.32
A variety of reasons for the decline in stroke mortality have been postulated, including the more aggressive treatment of hypertension. No one has suggested that the decline of stroke mortality might be related to the increasing use of carotid endarterectomy, as illustrated in Figure 18-1. Although this is not proof of a relationship, a possible relationship cannot be discounted.
In a review of the literature, Mentzer and colleagues33 identified 263 reported cases of stroke in evolution managed conservatively. Twenty-three percent had complete resolution or mild neurologic deficit on follow-up. Sixty-two percent had a moderate to severe deficit in the early recovery phase. The overall mortality was 14.5%. In their own series, 26 patients with stroke in evolution were treated conservatively. Mortality was 15%, but, more importantly, 66% suffered moderate to severe permanent neurologic deficits, with only five patients recovering completely or experiencing only mild neurologic dysfunction. These results are compared with a series of 17 patients operated on emergently for stroke in evolution. None of these patients had worsening of the preoperative neurologic deficit, four (24%) remained unchanged, and 12 (70%) had complete recovery.33
In 1972, Millikan reviewed the natural history of patients with progressing stroke.34 Of 204 patients, 12% were normal at 14 days, 7% had developed moderate to severe neurologic deficits, and 14% had died. Thus, stroke in evolution treated conservatively carries a poor prognosis. More than half of patients develop a severe permanent neurologic deficit within a few days of the onset, and approximately 15% die as a result. Only 10% to 20% recover full or partial neurologic function.34
Patients who experience TIAs are also at a higher risk of developing a stroke. In the Mayo Clinic population study,35 118 patients with TIAs were monitored as a control group without therapy. The stroke rates at 1, 3, and 5 years were 23%, 37%, and 45%, respectively. Most permanent deficits occurred during the first year. This represents a sixteenfold increased risk of stroke compared with an age- and sex-adjusted population. The Oxfordshire project reported an actuarial risk of stroke during the first year after the onset of TIAs to be 11% to 16%.36 For each subsequent year, the rate was 5% to 9% per year. Some series have reported lower figures,37,38 but the average reported in the literature is on the order of 30% to 35% at 5 years, or 10% the first year and 6% each year thereafter.
Finally, Toole39 noted that cerebral infarction in TIA patients goes unrecognized by either patient or physician surprisingly often. These lesions are now identified by better neuroimaging techniques, and there is now evidence to suggest that TIAs are actually small strokes.39 If a TIA is actually a small stroke, the implied benignity of TIAs must be reexamined, and it may be equally important to prevent TIAs. This consideration is further strengthened by the observations of Grigg and colleagues,40 who correlated cerebral infarction and atrophy as a function of TIAs and percentage of stenosis. They graded carotid stenosis in symptomatic patients from A (no stenosis) to E (occlusion). In patients with amaurosis fugax, the incidence of cerebral infarction rose from 2% in patients with stenosis grades A, B, and C to 40% in grade D and 58% in grade E. The incidence of atrophy increased in parallel, from 10% in grade A to 30% in grade E.
As noninvasive studies developed, detection of hemodynamically significant lesions in the carotid system improved. Kartchner and McRae monitored 1130 patients who either were asymptomatic or had nonhemispheric symptoms.41 The mean interval was 24 months. Of 303 patients with hemodynamically significant lesions, 11.9% had strokes at 2 years. The group with negative noninvasive studies had a much lower stroke rate, on the order of 3% over the same follow-up period. Busuttil and colleagues42 noted an unfavorable trend toward higher stroke rates in asymptomatic patients with hemodynamically significant lesions in the carotid bifurcation.
In a report by Roederer and colleagues,43 167 asymptomatic patients with cervical bruits were monitored with serial duplex scanning regardless of the degree of stenosis at the time of presentation. During follow-up, 10 patients became symptomatic. The development of symptoms was accompanied by disease progression in 80% of patients. By life-table analysis, the annual rate of symptom occurrence was 4%; however, the presence of progression graded at 80% stenosis was highly correlated with the development of either total occlusion of the internal carotid artery or new symptoms. Thus, 89% of the symptoms were preceded by progression of the lesion to greater than 80% stenosis. Progression of a lesion to more than 80% stenosis was an important warning sign, because it carried a 35% risk of ischemic symptoms or internal carotid occlusion within 6 months and a 46% risk at 12 months. Conversely, only 1.5% of the lesions that remained at less than 80% stenosis developed such a complication. These data suggest that careful follow-up with repeated noninvasive evaluation is of great assistance in determining the appropriate management of asymptomatic carotid lesions.43
In an analysis of 294 asymptomatic and nonhemispheric patients submitted to cerebrovascular testing, Moore and colleagues44 found a 15% stroke incidence during the first 2 years in patients with greater than 50% stenosis. In contrast, there was a 3% stroke incidence at 2 years in patients with 1% to 49% stenosis. The difference was found to be statistically significant (p < 0.05). The 5-year cumulative stroke incidence was 21% with greater than 50% stenosis, 14% with 1% to 49% stenosis, and 9% in patients with no noninvasive evidence of carotid artery disease.
Chambers and Norris45 monitored a group of 500 asymptomatic patients with noninvasive studies and clinical evaluation. They identified two high-risk groups: those with stenosis greater than 75%, and those who showed disease progression between studies. For patients with greater than 75% stenosis, the 1-year neurologic event rate (TIA and stroke) was 22%. The 1-year stroke rate alone was 5%. In a later publication,46 the authors continued to note that neurologic events correlated with an increasing percentage of stenosis as well as disease progression between test intervals. In the study viewed over 5 years, the annual average neurologic event rate was 10% to 15%, with the highest event rate occurring within the first year of diagnosis. Finally, the incidence of silent cerebral infarction as documented by computed tomography (CT) was studied in the same patient population. The authors noted a 10% incidence of cerebral infarction among patients with mild (35% to 50%) stenosis, 17% with moderate (50% to 75%) stenosis, and 30% in patients with severe (>75%) stenosis. The authors concluded that silent cerebral infarction might be an indication for carotid endarterectomy in asymptomatic patients.47
Other studies have suggested that the composition of the plaque influences the stroke risk of carotid artery lesions. In one analysis, 297 patients with carotid stenosis greater than 75% at the time of initial study were at higher risk than peers without significant narrowing or development of symptoms ipsilateral to the lesion.48 Even those patients with less than 75% stenosis were at greater risk if the associated plaque was less organized (i.e., soft). This was determined by B-mode ultrasonography, which was used to classify plaques as dense, calcified, or soft. A definite trend toward higher risk was seen in plaques of lower density. Only 10% of those patients with calcified plaque in significantly stenotic vessels developed symptoms, whereas 92% of patients with soft plaques and tight stenosis developed symptoms within the first 3 years of follow-up.48 The morphology of the atherosclerotic plaque, as documented by B-mode ultrasonography, is emerging as one of the more important factors associated with embolic potential and stroke risk. Two studies have concluded that a heterogeneous plaque carries an increased risk of stroke and is a variable independent from carotid stenosis alone.49,50
More recently, attempts have been made to better characterize the asymptomatic patient who is at higher risk for a cerebral ischemic event in order to identify subgroups of patients who would benefit from carotid endarterectomy compared to patients with carotid stenosis who are at low risk for an ischemic event. In addition to percent stenosis and plaque composition, two factors have been identified as being associated with increased stroke risk in the asymptomatic patient. These factors include the presence of silent brain infarction as seen on a screening CT scan and silent brain embolic signals as documented using transcranial Doppler. Kakkos and colleagues51 studied 821 patients with CT scans. In patients with 60% to 99% stenosis and without silent brain infarction on CT, the annual rate of TIA and stroke was 1.3%. In patients with silent brain infarction, the annual event rate was 4.4%, suggesting that this subgroup of patients might benefit from carotid endarterectomy, leaving the low-risk group free from an unnecessary intervention.51 Markus and colleagues52 evaluated 467 asymptomatic patients with high-grade carotid stenosis in a multicenter study. Transcranial Doppler identified 77 patients with embolic signals. The annual risk of TIA and stroke was 7.13% in patients with embolic signals compared to 3.04% in patients without embolic signals. For ipsilateral stroke alone, the hazard ratio was 6.37. These findings suggest that screening for silent brain emboli is another method for identifying a high-risk group who are more likely to benefit from intervention.52
The embolic potential of ulcerated carotid lesions has been well documented.53–55 Patients who experience symptoms from these lesions probably have the same prognosis as patients with occlusive lesions. Whether the former patient group responds more favorably to platelet antiaggregants remains to be determined. Moore and coworkers56 first pointed out that asymptomatic patients with significant ulceration in a carotid plaque in the absence of stenosis appear to be at higher risk of stroke.56 In a subsequent report, they expanded their series to 153 patients with asymptomatic, nonstenotic ulcerative lesions in the carotid bifurcation. Patients with deep (grade B) or complex (grade C) ulcerations received follow-up and were found to have a stroke rate of 4.5% and 7.5% each year, respectively.57 Other reports have suggested a similar stroke risk for complex ulcerations in the carotid bulb. However, a much lower stroke risk was reported for deep (grade B) ulcerations, with no significant added risk of stroke observed in these patients. Controversy still exists about deep ulcerations without complex morphology. However, agreement exists that complex ulcerations in the carotid bulb increase the risk of stroke in asymptomatic patients.58
The presence of an asymptomatic hemodynamically significant stenosis may increase the risk of stroke during major surgery. Kartchner and McRae reported their experience with 234 patients, 41 of whom had evidence of significant carotid artery stenosis by oculoplethysmography.59 Seven postoperative strokes developed in the group with positive criteria (17%), whereas postoperative cerebral infarction developed in 2 of 192 patients (1%) with negative noninvasive studies. The mechanisms of stroke and the territory involved were not specifically reported. This high incidence of permanent neurologic deficits led the authors to conclude that prophylactic carotid endarterectomy should be considered in patients with hemodynamically significant carotid stenosis who are undergoing a major cardiovascular procedure.60
Other series have reported results to the contrary.60–63 Using noninvasive vascular evaluation and, in one series, angiography, patients with 50% or greater stenosis in the carotid bifurcation were compared with patients who had lesser degrees of stenosis undergoing cardiovascular surgery. No increased incidence of perioperative strokes was found in patients with positive criteria. Most of these investigators, however, excluded preocclusive stenosis in their considerations. Lesions causing 90% or greater stenosis were excluded from these series and were subjected to prophylactic endarterectomy before cardiovascular operation.
Cardiac surgeons have long been concerned about the presence of carotid stenosis in patients who will be undergoing cardiopulmonary bypass. Their concern is that during bypass there will be a decrease in pump perfusion pressure and a corresponding and unacceptable drop in cerebral blood flow. In fact, the opposite occurs. Von Reutern and colleagues64 used transcranial Doppler ultrasonography to study middle cerebral artery blood flow before and during cardiopulmonary bypass in patients with and without carotid artery disease. Surprisingly, middle cerebral artery blood flow actually increased during cardiopulmonary bypass. Although the increase was not as great in patients with carotid artery disease, it was clearly an increase over baseline. This observation should dispel concern about the potential drop in cerebral blood flow in patients with carotid stenosis while using the pump.
Patients with a combination of severe carotid stenosis and symptomatic coronary artery disease represent a cohort that is at high risk of death, MI, and stroke. Brener and colleagues65 performed an extensive literature review that examined complications associated with different treatment strategies. Patients who underwent staging with carotid endarterectomy first had a high cardiac morbidity and mortality. Patients who had coronary bypass first had a higher stroke morbidity. The data suggested that combined or simultaneous coronary artery bypass grafting and carotid endarterectomy might reduce overall morbidity and mortality. However, evidence from retrospective reviews was not sufficiently compelling to make a definitive recommendation.65 Consensus exists that this is an appropriate topic for a prospective, randomized trial.
Pathology of Extracranial Arterial Occlusive Disease
Atherosclerosis
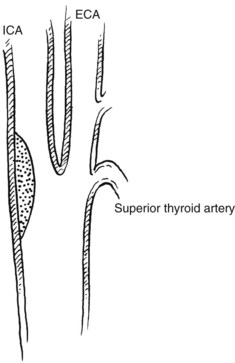
The carotid bifurcation appears to be susceptible to the development of atherosclerotic plaques.66 Frequently, severe changes at the carotid bifurcation occur with minimal or no changes present in the common or internal carotid artery.67 Several investigators have proposed conflicting theories based on hemodynamic observations in various models. High shear stress and fluctuations in shear stress,68 disordered or turbulent flow, flow separation, and high and low flow velocity have all been implicated.69–72 Which of these mechanisms is responsible for plaque formation is not known. Zarins and colleagues73 used a model of the human carotid bifurcation under steady flow and compared its hemodynamics with those of cadaver specimens. They concluded that carotid lesions localize in regions of low flow velocity and flow separation rather than in regions of high velocity and increased shear stress. They used their model to explain the propensity of the outer wall of the carotid sinus opposite the flow divider to develop atherosclerotic plaques (Figure 18-2). This may have further clinical implications, in that an enlarged carotid bulb after endarterectomy may create a region of reduced flow velocity and increased boundary layer separation, which may favor recurrent plaque deposition.
Once the initial intimal injury is produced by these forces, platelet deposition, smooth muscle cell proliferation, and the slow accumulation of lipoproteins are involved in the reparative process (Figure 18-3). These eventually lead to plaque formation, which further alters the hemodynamics of the system and favors further injury.
The contribution of platelets to atheroma development can take several forms.74 Platelets may adhere to one another, to the diseased vessel, or both; this can lead to thrombus formation. This process may narrow the vessel lumen, or the thrombus may dislodge, resulting in distal embolization. Vasoactive substances stored in granules within the platelet may be released, causing vasospasm and further contributing to compromise of the arterial lumen. The platelets’ interaction with collagen, exposed in an injured intima, may include elaboration of a smooth muscle growth factor that can lead to intimal thickening. The activation of enzymes in platelets, by their contact with collagen, initiates the production of highly active prostaglandins. The production of thromboxane A2 represents the final common pathway of platelet response to diverse stimuli.75 This substance is a potent stimulant of platelet aggregation and a powerful vasoconstrictor and is believed to be important in the pathophysiology of plaque formation or the development of symptoms from an already established atheroma.
Hemorrhage into a plaque may also play a significant role in the development of symptoms from an atherosclerotic lesion. Imbalances in wall tension secondary to asymmetrical deposition of plaques can lead to sudden plaque fracture and intraplaque hemorrhage.76 These can lead to sudden expansion of the atheroma, with acute restriction of flow or breakdown of the intimal surface and concomitant embolization. An alternative mechanism for sudden intraplaque hemorrhage may be related to an increase in neovascularity within the plaque substance. Hypertension may be responsible for precipitating rupture of neovascular vessels, leading to intraplaque hemorrhage and expansion.77 This process may be responsible for a large number of symptomatic lesions. In a prospective evaluation of 79 atheromatous plaques removed from 69 patients undergoing carotid endarterectomy, 49 of 53 (92.5%) symptomatic patients had evidence of intramural hemorrhage.78 In contrast, only 7 of 26 (27%) asymptomatic patients showed recent or acute intraplaque hemorrhage. Rupture of an atherosclerotic plaque with intraluminal release of atheromatous debris has also been correlated with acute stroke and internal carotid occlusion in an autopsy study.79
Fibromuscular Dysplasia
Fibromuscular dysplasia is a nonatherosclerotic process that affects medium-size arteries. It was first described in the carotid artery in 1964,80 and since then it has been recognized as a cause of cerebrovascular symptoms.81 It may also affect the intracranial arteries, and approximately 30% of patients with cervical involvement have associated intracranial aneurysms.82 Up to 65% of patients have bilateral disease,82 and 25% have associated atherosclerotic changes.83
Four histologic types of fibromuscular dysplasia have been described84:
1. Intimal fibroplasia accounts for about 5% of cases and affects both sexes equally. It usually appears as long tubular stenoses in young patients and as focal stenoses in older patients. It results from an accumulation of irregularly arranged subendothelial mesenchymal cells with a loose matrix of connective tissue. Medial and adventitial structures are always normal.
2. Medial hyperplasia is a rare form of the disease that produces focal stenoses. The intima and adventitia remain normal, whereas the media shows excess smooth muscle.
3. Medial fibroplasia is the most common pattern of fibromuscular dysplasia, accounting for most, if not all, internal carotid artery involvement. It may appear as a focal stenosis or multiple lesions with intervening aneurysmal outpouchings. Histologically, the disease is limited to the media, with replacement of smooth muscle by compact fibrous connective tissue. The inner media may show an accumulation of collagen and ground substance separating disorganized smooth muscle cells. Gradation of these changes correlates with the severity of the lesion. Mural dilatations and microaneurysms are common.
4. Perimedial dysplasia is characterized by the accumulation of elastic tissue between the media and adventitia. It affects renal arteries and is associated with macroaneurysms.
Fibromuscular dysplasia preferentially affects long arteries with few primary branches. Hormonal effects on medial tissue, mechanical stresses on the vessel wall, and unusual distribution of the vasa vasorum in these arteries seem to play a causative role.84 Some experimental evidence85 and the fact that women are most commonly affected (92% of cases)83 support a possible role of hormones in this process. In the appropriate hormonal environment, the normal paucity of vasa vasorum in long, nonbranching arterial segments such as the extracranial carotid artery and the renal artery may predispose to mural ischemia and initiation of the fibroplastic process. Experimental evidence supports this concept.86
The exact cause of symptoms is controversial. Thromboembolism from clot, platelets, or both; decreased flow owing to a critical stenosis or a series of noncritical narrowing; intracranial involvement, with or without aneurysm formation; and hypertension have been implicated.83
Coils and Kinks
Coils and kinks of the extracranial system on occasion have been associated with fibromuscular dysplasia.83 More commonly, these are due to embryologic events and changes that occur in the aging process. Neurologic manifestations from these anomalies have been reported in children87 and in adults.88–90 Embryologically, the internal carotid artery is derived from the third aortic arch and the dorsal aortic root. In the early stages of development, a normally occurring kink is straightened as the heart and great vessels descend in the mediastinum. Failure of this process may account for the occurrence of coils and loops in children and for its bilaterality in approximately 50% of cases.90
In adults, kinking of the extracranial vessels is almost always associated with atherosclerosis. In the aging process, loss of elasticity of the vessel wall occurs, which, in combination with lateral stresses, causes elongation between fixed points—the skull and the thoracic inlet. This produces bowing, with the eventual formation of coils and kinks. Between 5% and 16% of patients submitted for angiographic evaluation have coiling or kinking of one of the extracranial vessels.88,90 Kinking of the artery is more likely to produce symptoms because of either flow reduction or concomitant plaque formation with distal embolization. Kinking is considered to be an angle of less than 90 degrees between arterial segments (Figure 18-4). Flow restriction is unlikely to exist in the absence of this configuration. This acute angulation is more likely to occur when the head is turned to the ipsilateral side.87 In other cases, contralateral rotation, neck flexion, and extension may exaggerate the abnormality, leading to markedly reduced flow. A history of TIAs associated with head motion should lead the clinician to suspect the presence of a kink. Abnormal pulsations in the neck, sometimes suggesting an aneurysmal dilatation, may be present on physical examination. Secondary arteriosclerotic changes can occur because of abnormal flow patterns that predispose to plaque formation and ulceration, accounting for the development of neurologic symptoms. Rarely is the vertebral circulation affected by a kink.88
Aneurysms
Aneurysms of the carotid artery can cause neurologic symptoms by several mechanisms. Thrombosis and rupture are rare, but embolization is a frequent event.91 Pressure on cranial nerves can be seen when expansion is rapid, but more frequently this is associated with acute dissection.
Most extracranial aneurysms are secondary to atherosclerosis. Internal elastic lamina disruption and medial thinning are frequent histologic findings.92 Two types of aneurysms are recognized: fusiform and saccular. Fusiform aneurysms are more common; they are frequently bilateral and are associated with other arterial aneurysms. Saccular aneurysms are often unilateral and tend to involve the common or internal carotid arteries more often. They may also have a congenital, degenerative, or traumatic origin.93 Atherosclerotic aneurysms of the extracranial circulation are almost always associated with hypertension.92
Trauma is a frequent cause of carotid aneurysms. These are usually saccular and most commonly result from blunt rather than penetrating injury. Hyperextension and rotation of the neck cause compression of the internal carotid artery on the transverse process of the atlas.94 An intimal injury is produced that frequently leads to thrombosis, but it can also produce aneurysmal dilatation.92
Mycotic aneurysms are rare. Syphilis and peritonsillar abscess were once common causes of these aneurysms.95 Staphylococcus aureus is currently the predominant responsible organism.
False aneurysms of the carotid artery may form after penetrating injury, but the most frequent cause is previous carotid surgery. These aneurysms are more common after patch closure of the artery than after primary closure.91 Disruption of the suture line by infection, suture failure, and technical error are believed to be responsible for their formation. False aneurysms can expand, thrombose, rupture, or lead to distal embolization. The diagnosis of false aneurysm is an indication for surgical repair.
Acute dissection of the carotid artery with or without aneurysm formation is another cause of neurologic events resulting from abnormalities in the extracranial circulation. It can occur secondary to atherosclerosis, fibromuscular dysplasia, or cystic medial necrosis.96 A history of trauma may or may not be present. On gross inspection, a sharply demarcated transition between the normal color and size of the carotid artery and the dark blue, cylindrical dilatation in the dissected segment is noted.97 More commonly, the internal carotid artery is affected, and frequently the end of the dissection is not surgically accessible. A double lumen is usually present, with the dissection occurring in the outer layers of the media. Smooth muscle cells are widely separated, and degeneration and fragmentation of the internal elastic membrane occur.97 The most frequent presentation is a sudden onset of temporal headache or cervical pain associated with a neurologic or visual deficit or Horner syndrome. Acute expansion may cause compression of cranial nerves IX, X, XI, or XII, with concomitant dysfunction.96 Horner syndrome is thought to be secondary to disruption of periadventitial sympathetic fibers. The carotid artery is far more frequently affected than the vertebral artery. Only a few cases of the latter have been reported, with involvement of the segment between C1 and C2 noted consistently.96
Takayasu Arteritis
In 1908, Takayasu described ocular changes in a 21-year-old woman with nonspecific arteritis.98 These changes consisted of a peculiar capillary flush, with rustlike arteriovenous anastomoses around the papilla and blindness owing to cataracts. Similar cases were later described with the absence of pulses in the arm. Since then, Takayasu arteritis has been recognized as a cause of neurologic symptoms secondary to a nonspecific inflammatory process of unknown cause segmentally affecting the aorta and its main branches. The end result of this process is constriction or occlusion of, and occasional aneurysm formation in, the affected vessels secondary to marked fibrosis and thickening of the arterial wall.99 Originally thought to be rare in the Western hemisphere, many cases of atypical coarctations of the aorta and other unusual lesions of its main branches are now well recognized as Takayasu arteritis. This explains the many eponyms given to this syndrome.100
Four varieties of the disease are recognized.101 In type 1, involvement is localized to the aortic arch and its branches. Type 2 does not have arch involvement; the lesions are confined to the descending and abdominal aorta. Type 3 has features of both, and type 4 describes any of the first three types plus involvement of the pulmonary artery. In a retrospective study of 107 patients, 84% were female, and 80% were aged 11 to 30 years.101
Other forms of arteritis, specifically giant cell arteritis, can cause neurologic symptoms because of extracranial or intracranial involvement. These patients are older than those with Takayasu arteritis, and both sexes appear to be equally affected.100 Systemic symptoms are usually present. Tenderness over the carotid artery or other affected areas may occur.102 The histologic picture is characteristic, with changes confined to the media, where a large number of giant cells interspersed with lymphocytes are seen. Early diagnosis is important, because corticosteroid therapy may abort the latter stages of the process.102
Radiation Therapy Injury
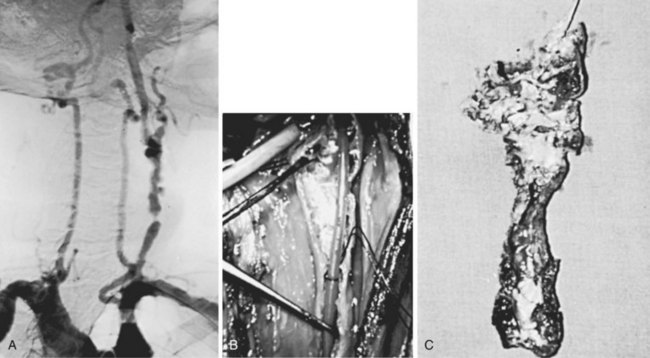
External cervical radiation therapy is recognized as a cause of accelerated atherosclerotic changes in the extracranial circulation. Experimentally, atherosclerotic lesions similar to the naturally occurring ones can be produced in the abdominal aorta in dogs by x-ray and electron beam radiation.103 Injury to the endothelial cell, ground substance, elastic lamina, and smooth muscle appears to alter the vessel wall, increasing its permeability to circulating lipids and impairing its ability to repair elastic tissue, leading to the formation of a plaque characterized by fibrosis, fatty infiltration, and intimal destruction.104 These changes can occur months to years after completion of therapy. Lesions occur in locations unusual for atherosclerosis (Figure 18-5). Blowout of the affected carotid artery may occur, but this is more frequent when surgery is combined with radiation in treating cervical malignancies. Hyperlipidemia and hypercholesterolemia appear to predispose patients receiving radiation therapy to the development of these accelerated changes.105 Endarterectomy of the affected segments is difficult but can be performed safely.104,106
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree