Excision of Hourglass Tumors of the Paravertebral Sulcus
A. John Popp
Makoto Goda
Darryl J. DiRisio
Darroch W. O. Moores
Hourglass tumors of the thoracic paravertebral sulcus present with a constellation of anatomic, pathologic, and surgical considerations that make their treatment unique. The appellation hourglass tumor refers to a lesion with an intraspinal component and an intrathoracic component connected by a narrow waist of tumor, the growth of which has been restricted by the confines of the bony intervertebral foramen. In the series of 706 neurogenic tumors of the thoracic paravertebral region reported by Akwari and colleagues,1 nearly 10% were found to have extension into the spinal canal. In patients where the source of the tumor was reported, 68% were nerve sheath in origin, 30% originated from the sympathetic chain, and 2% arose from paraganglion cells. Overall, 10% of the neurogenic hourglass tumors were malignant. In addition to neurogenic lesions, a number of other mesenchymal tumors—such as hemangiomas, other blood vessel tumors, and lipomas—also may grow into the spinal canal when they originate in the paravertebral sulcus.
Anatomic Considerations
The anatomic features that influence the surgical approach to hourglass lesions of the thoracic spine include the proximity of the pleural cavity, the presence of ribs, and the osseous and neural anatomy.
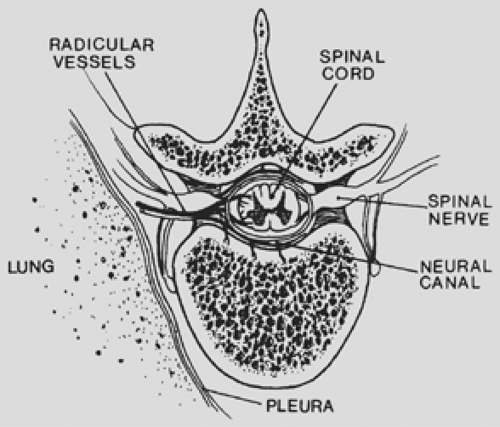
The thoracic spinal column contains 12 segments. The spinal canal is delimited ventrally by the vertebral bodies and the interposed discs and dorsally by the spinous processes, paired laminae, facet joints, and pedicles. Twelve paired ribs articulate with the vertebral bodies at the diarthrodial joints. The intervertebral foramen (Fig. 197-1) is formed cephalad and caudad by the pedicles, dorsally by the superior and inferior articular facets, and ventrally by the contiguous vertebral bodies and interposed disc.
The spinal cord and the proximal portion of the emerging nerve roots are contained within the spinal canal (Fig. 197-2). Surrounding the neural tissues are three layers of the meninges: the dura mater, arachnoid, and pia mater. The arachnoid envelope contains cerebrospinal fluid. The subarachnoid space extends a variable distance along the nerve root; thus, if the nerve root must be sacrificed during tumor resection, unrecognized arachnoid injury may result in a spinal-pleural cerebrospinal fluid fistula.
Radicular arteries enter the intervertebral foramina to perfuse the intraspinal structures. Spinal medullary branches arise from the radicular arteries to supply the spinal cord. Radicular branches are random and do not enter every intervertebral foramen in the thoracic region. Attempts should be made to preserve the radicular blood supply, although sacrifice of a radicular artery might be necessary to fully resect an hourglass tumor, thereby threatening circulation to the spinal cord. Despite this concern, neural impairment secondary to spinal cord infarction caused by vascular sacrifice rarely occurs, perhaps because most hourglass tumors are slow growing and the resultant gradual occlusion of important nutrient vascular channels allows for the development of collateral circulation.
The complexity of osseous, vascular, and neural anatomy encountered in the thoracic region complicates the operative approach to hourglass tumors. Unlike the lumbar region, where the spinal cord is absent, the presence of the spinal cord in
the thoracic region renders a posterior approach to the spinal canal unsafe. Unlike the cervical region, where the presence of the spinal cord also requires careful planning of the operative approach, the presence of ribs and the pleura in the thoracic region complicate the surgical exposure.
the thoracic region renders a posterior approach to the spinal canal unsafe. Unlike the cervical region, where the presence of the spinal cord also requires careful planning of the operative approach, the presence of ribs and the pleura in the thoracic region complicate the surgical exposure.
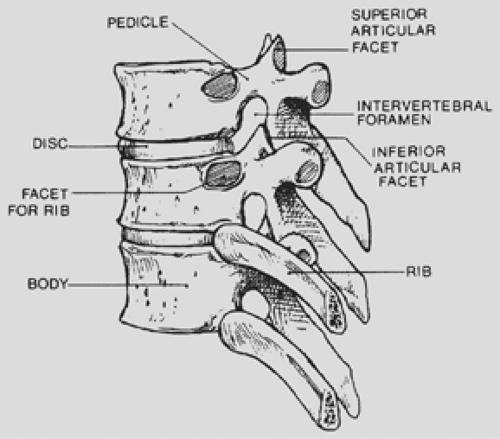 Figure 197-1. Lateral view of a segment of the thoracic spine demonstrating the intervertebral foramen. |
Clinical Presentation
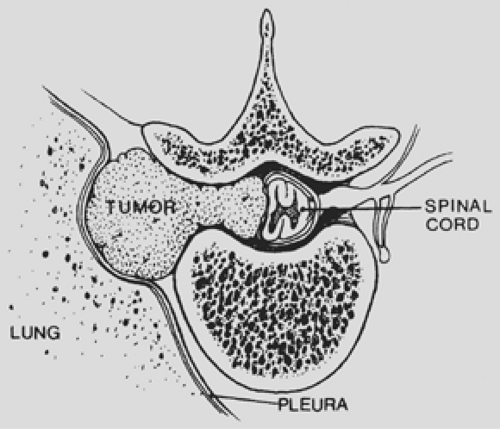
An hourglass tumor may be asymptomatic and found on routine chest radiography (Fig. 197-3) or may present with either pulmonary or neurologic symptoms. Akwari and colleagues1 noted that approximately one-third of the hourglass tumors were asymptomatic. Tumors with a large intrathoracic component may produce shortness of breath or cough. Neurologic deficits may be caused by spinal cord compression or involvement of nerve roots (Fig. 197-4). Patients with myelopathy may present with gait difficulty, urinary and fecal incontinence, and loss of sensory and autonomic function below the level of the lesion. Those with radicular symptoms may present with radicular pain at the level of the tumor; on examination, altered sensation may be found in a dermatomal distribution. Rarely, patients with an upper thoracic tumor may present with Horner’s syndrome due to compression of the sympathetic chain. The clinical manifestations of this syndrome include pupillary miosis, eyelid ptosis, and anhydrosis of the face and neck ipsilateral to the lesion.
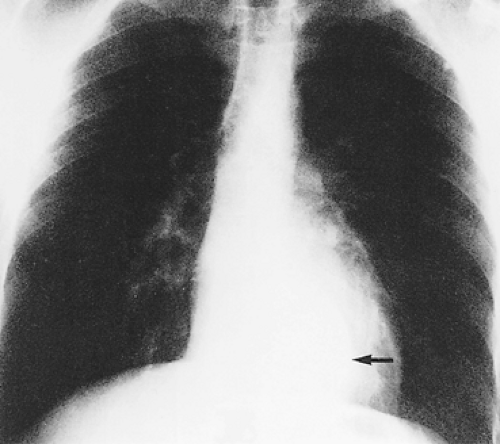 Figure 197-3. Posteroanterior chest radiograph. Arrow indicates location of an asymptomatic hourglass neurogenic tumor. |
Diagnostic Investigation
All patients being investigated for a paravertebral mass must also be evaluated for intraspinal extension. Assessment begins with a history and physical examination followed by diagnostic studies that may include plain radiographs of the thoracic spine, coned-down computed tomographic (CT) scan, magnetic resonance imaging (MRI), and CT myelography. Imaging used to evaluate patients with hourglass lesions has evolved considerably since the late 1980s with the introduction of high-resolution CT scanning and MRI. Prior to the development of newer imaging modalities, diagnosis depended on using plain radiography to identify an enlarged or eroded neural foramen in a patient with a paravertebral tumor. CT myelography is still occasionally used to define the intraspinal component of the tumor, but MRI (Fig. 197-5) has become the single best examination to disclose the characteristic shape and extent of the tumor. Spinal angiography is used occasionally to add information about blood supply to the tumor and spinal cord, particularly with lower thoracic tumors, when perfusion by the artery of Adamkiewicz becomes more likely. However, the risk for spinal angiography outweighs any potential benefit in most instances.
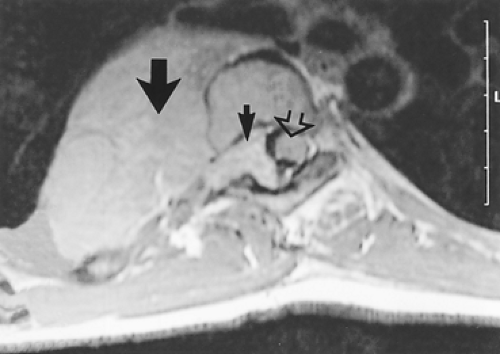 Figure 197-5. MRI demonstrates narrow waist of tumor connecting large intrathoracic neurofibroma (large arrow) with smaller intraspinal component (small arrow). Note enlargement of the neural foramen and the displaced spinal cord (open arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|