Diuretics are among the most commonly prescribed cardiovascular (CV) medications. The strength of evidence supporting the effectiveness of diuretics in lowering blood pressure and for preventing major adverse CV events in patients with hypertension varies considerably among diuretic classes and even among agents within the same class. Unfortunately, common prescribing habits among American physicians, including specialists in CV diseases, are not in line with the existing evidence regarding diuretic therapy for improving CV prognosis. In conclusion, although hydrochlorothiazide is the standard diuretic used for hypertension, the outcomes data suggest that chlorthalidone, indapamide, and possibly even the aldosterone receptor blockers (spironolactone and eplerenone) may be superior agents.
In this report, we review published data on the effects of various diuretics on major clinical outcomes in patients with hypertension. As a practical matter, all the agents discussed in this review are widely available as generics, and a 30-day supply of most can be purchased for $4. Thus, cost should not be an issue when choosing the most effective diuretic for patients with hypertension, even those who are uninsured.
Thiazide Diuretics
The thiazide designation collectively encompasses a group of thiazide derivatives (hydrochlorothiazide, chlorothiazide, and others) and thiazide-like agents (chlorthalidone, indapamide, and metolazone) that principally act on the distal convoluted tubules and inhibit the sodium chloride transporter. Indapamide exerts some of its diuretic and antihypertensive effects through additional mechanisms that are not yet fully elucidated.
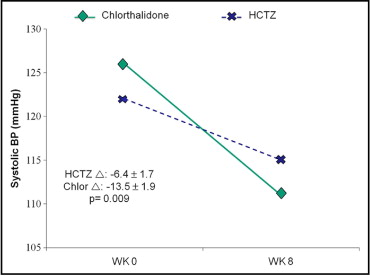
Since their advent, thiazide diuretics have been a cornerstone in hypertension treatment. However, thiazide diuretics vary considerably in their blood pressure (BP)–lowering potency. For example, chlorthalidone has a longer half-life and a larger volume of distribution compared to hydrochlorothiazide. Therefore, it is 1.5 to 2 times more potent than hydrochlorothiazide and yields a larger and more sustained reduction in systolic BP ( Figure 1 ), and the 2 agents are not equivalent in their antihypertensive properties at low doses.
The efficacy of thiazide diuretics as first-line therapies for preventing mortality and morbidity in patients with hypertension has been established. Furthermore, thiazide diuretics are as effective as many other classes of BP-lowering medications in preventing coronary heart disease (CHD) and stroke for a given reduction in BP. Hydrochlorothiazide, chlorthalidone, and indapamide are the thiazide diuretics most commonly used in clinical practice and most widely studied in major clinical trials. We herein will summarize and critically review clinically trials supporting the role of these diuretics in improving cardiovascular (CV) prognosis in patients with hypertension.
Hydrochlorothiazide and chlorthalidone
The interchangeability of hydrochlorothiazide and chlorthalidone has been a matter of intense debate. This stems not only from differences in their pharmacokinetics but also from the strength of the evidence base supporting their utility in improving clinical outcomes.
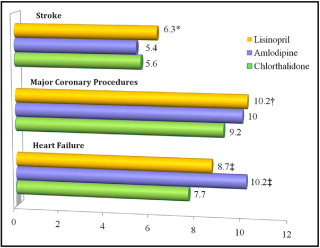
Studies with chlorthalidone have demonstrated a 17% reduction in all-cause mortality, a 36% reduction in stroke, a 33% reduction in total CV events, and 41% reduction in fatal and nonfatal congestive heart failure (CHF) compared to placebo. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) demonstrated chlorthalidone to be noninferior to lisinopril or amlodipine in preventing fatal and nonfatal CV events as well as total mortality, and it was more effective in preventing CHF and major CHD procedures than either comparator ( Figure 2 ). However, some editorials were critical of the design of ALLHAT, citing potential biases favoring diuretics. Yet the remarkable benefits of chlorthalidone in this large randomized, double-blind, multicenter trial sponsored by the National Heart, Lung, and Blood Institute formed the basis for the recommendation that a thiazide diuretic should be used as an initial therapy in almost all patients with hypertension, according to the seventh report of the Joint National Committee on the detection, prevention, and treatment of hypertension.
The evidence supporting the use of hydrochlorothiazide is less consistent. Hydrochlorothiazide plus triamterene resulted in a 38% reduction in CV mortality compared to placebo in patients with hypertension aged >60 years. Hydrochlorothiazide plus amiloride was significantly more effective than atenolol plus placebo in preventing stroke, total CHD events, and all-cause mortality. The same combination of hydrochlorothiazide and amiloride was compared to nifedipine in the International Nifedipine GITS Study: Intervention as a Goal in Hypertension Treatment (INSIGHT), which showed that the 2 treatments were equally effective at reducing fatal and nonfatal CV events.
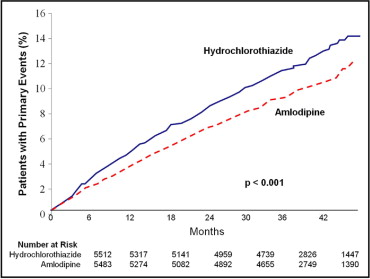
However, in a randomized trial, the Oslo Study, hydrochlorothiazide (compared to no treatment) did not improve overall mortality or total CV events in asymptomatic patients with hypertension, despite lowering BP by 17/10 mm Hg. Although a reduction in the incidence of stroke was observed in the hydrochlorothiazide arm, it was, unfortunately, offset by an increase in CHD events, which the investigators attributed to unfavorable metabolic effects of active treatment (95% of patients received hydrochlorothiazide). The Second Australian National Blood Pressure Study (ANBP-2) group showed that hydrochlorothiazide was inferior to enalapril for lowering the risk for a composite of overall mortality and all CV events, which was driven mainly by a higher rate of myocardial infarction in the hydrochlorothiazide arm. More recently, in a group of >11,000 high-risk patients with hypertension taking benazepril, the group randomized to hydrochlorothiazide had 20% more adverse CV events than the group randomized to amlodipine ( Figure 3 ). This was observed despite a similar reduction in ambulatory and office visit BP measurements in the hydrochlorothiazide and amlodipine arms.
There are a number of potential explanations for this lack of consistency with hydrochlorothiazide for improving outcomes, including study design flaws. However, in all studies that showed benefit with hydrochlorothiazide, a second diuretic was used in combination, raising the possibility that hydrochlorothiazide alone is inadequate for optimizing CV prognosis. The prescribed dose of hydrochlorothiazide might also be an important factor in this regard, because the lack of benefit with hydrochlorothiazide has been generally seen in studies in which lower doses were used (<25 mg/day). Yet hydrochlorothiazide at these lower doses induced BP reductions that were equivalent to those seen with the comparators and still resulted in less than expected clinical benefit. Moreover, using hydrochlorothiazide at higher doses (50 to 100 mg/day) was associated with a higher incidence of hypokalemia, which has been linked to increased rates of cardiac arrest and type 2 diabetes mellitus.
Head-to-head comparison between hydrochlorothiazide and chlorthalidone in an outcomes-based clinical trial has not been performed and might never be done, because the 2 medications are available generically. The best evidence for the superiority of chlorthalidone to hydrochlorothiazide comes from nonrandomized comparison of the 2 agents in the Multiple Risk Factor Intervention Trial (MRFIT), in which hydrochlorothiazide use was associated with excess CHD and all-cause mortality compared to chlorthalidone. This trend was reversed after the trial’s steering committee decided to switch from hydrochlorothiazide to chlorthalidone at all participating sites. A meta-analysis comparing the efficacy of different thiazide-based antihypertensive therapies showed no difference in major health outcomes between chlorthalidone-based and other thiazide-containing regimens. The technique used in this analysis provides indirect comparison of these agents to each other through comparing each of them to placebo. This statistically complex yet valid technique is used when clinical trials providing direct comparison between therapies of interest are lacking. It is, however, fraught with several limitations.
Despite the data suggesting stronger and more consistent lowering of BP and lower risk for adverse CV outcomes for chlorthalidone compared with hydrochlorothiazide, the latter agent continues to be the most prescribed diuretic in the United States. This preference for hydrochlorothiazide, at least by American clinicians, is likely due to many reasons, including clinical tradition, the widespread use of this agent in combination antihypertensive pills, and commonly recognized abbreviations (HCT and HCTZ). Hydrochlorothiazide is available in combination with a variety of popular antihypertensive agents compared with chlorthalidone, which is available only in combination with atenolol, clonidine, and reserpine, all of which have serious shortcomings. Hypokalemia is another concern with chlorthalidone, although the risk for serious hypokalemia is less problematic when using doses not higher than 12.5 to 25 mg/day. Combining chlorthalidone (or 1 of the other diuretics from this class) with another BP medication that can offset potassium loss, such as an angiotensin-converting enzyme inhibitor, an angiotensin receptor blocker, or an aldosterone receptor blocker, will not only potentiate BP lowering but also mitigate the risk for hypokalemia.
Thiazide diuretics have some limitations that are worth mentioning. For example, they lack vasculoprotective properties (increasing nitric oxide availability) and other non-BP-dependent vascular benefits possessed by some other antihypertensive drugs. In addition, concerns related to adverse effects and potential safety issues (collectively referred to by some investigators as the “dark side” of diuretics) have been raised. Hypokalemia, hyperuricemia, hyperglycemia, and exacerbation of metabolic syndrome are common side effects of thiazide diuretics. Focal glomerular and interstitial injury has also been seen in hydrochlorothiazide-treated rats. Moreover, prolonged exposure to medium or high doses of diuretics might be associated with an increased risk for renal cell carcinoma. However, the clinical significance of these potential liabilities of thiazide diuretics and their relevance to daily practice and choice of antihypertensive agents remain uncertain.
Indapamide
Despite the similarities to other members of the thiazide family, indapamide has unique features that render it a particularly efficacious and advantageous antihypertensive agent.
It produces a significant and sustained reduction in BP, and compared to other thiazide diuretics, it is associated with a lower incidence of serious hypokalemia and hyperglycemia. Furthermore, unlike other thiazide diuretics, indapamide retains efficacy in patients with chronic kidney disease.
The impact of indapamide on clinical outcomes has been studied in several large randomized controlled trials ( Table 1 ). Indapamide was associated with greater reduction in left ventricular mass and regression of left ventricular hypertrophy (LVH) compared to either enalapril or atenolol monotherapy. Additionally, indapamide was noninferior to enalapril in reducing urinary albumin excretion in patients with diabetes and hypertension, and the combination of indapamide with perindopril provided incremental renal protection over enalapril alone.
| Study | Agent | Comparator | End Point | Key Findings |
|---|---|---|---|---|
| PICXEL | IND + perindopril | Enalapril | Change in LVMI | Greater reduction in LVMI with IND/perindopril vs enalapril (−13.6 vs −3.9 g/m 2 , p <0.0001) |
| LIVE | IND | Enalapril | Change in LVMI | Greater reduction in LVMI with IND vs enalapril (−8.4 vs −1.9 g/m 2 , p <0.013) |
| REASON | IND + perindopril | Atenolol | LVM | Greater reduction in LVM with IND/perindopril vs atenolol (−13.6 vs −4.3 g, p = 0.027) |
| NESTOR | IND | Enalapril | Change in albuminuria | IND is noninferior to enalapril in reducing albuminuria (−46% vs −47%, p = NS) |
| PREMIER | IND + perindopril | Enalapril | Change in albuminuria | Greater reduction in albuminuria with IND/perindopril vs enalapril (−40% vs 27%, p = 0.007) |
| HYVET | IND ± perindopril | Placebo | Fatal or nonfatal stroke (primary end point), all-cause mortality, and CV mortality (secondary end points) | 30% reduction in stroke (p = 0.06), 21% reduction in all-cause mortality (p = 0.02), 23% reduction in CV mortality (p = 0.06), and 64% reduction in CHF (p <0.001) |
| ADVANCE | IND + perindopril | Placebo | Composite of macrovascular (cardiovascular death, nonfatal MI, or nonfatal stroke) and microvascular (new or worsening nephropathy or retinopathy) events | 9% RRR in primary end point (p = 0.04), 18% RRR in CV death (p = 0.03), 14% RRR in all-cause mortality (p = 0.03), and 21% RRR in total renal events (p <0.001) |
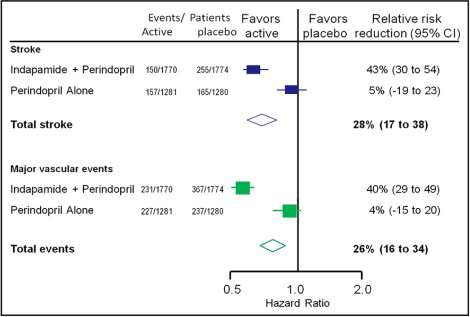
| PROGRESS | IND + perindopril | Placebo | Fatal or nonfatal stroke (primary end point), major CV events, CV or all-cause mortality (secondary end points) | IND + perindopril: 43% (95% CI 30% to 54%) reduction in stroke, 40% (95% CI 29% to 49%) reduction in major CV events |
| Perindopril alone: 5% (95% CI −19% to 23%) reduction in stroke, 4% (95% CI −15% to 20%) reduction in major CV events |
In the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), which enrolled 6,105 patients with histories of stroke or transient ischemic attack, treatment with perindopril with or without indapamide resulted in a 28% relative risk reduction in the risk for fatal and nonfatal stroke and a 26% reduction in major CV events relative to placebo. Notably, statistically significant reductions in stroke and major CV events were noted only in patients who received indapamide plus perindopril combination therapy, not in patients who received perindopril only. Those in the indapamide plus perindopril combination therapy group had superior reductions in BP compared to those in the perindopril-only group. This study suggests that the addition of indapamide to an angiotensin-converting enzyme inhibitor improves BP control and provides additional risk reduction in patients with hypertension and cerebrovascular disease compared to angiotensin-converting enzyme inhibitor monotherapy ( Figure 4 ). Unfortunately, however, PROGRESS did not include an indapamide monotherapy arm, precluding direct conclusions about its effects not in combination with perindopril.