Evaluation & Treatment of the Perioperative Patient
Sanjiv J. Shah, MD
Michael H. Crawford, MD
The prevalence of cardiovascular disease and the death rate associated with it rise sharply after age 45, an age when the incidence of noncardiac surgeries is also increasing, and approximately one-third of the 25 million surgical procedures done annually are performed in patients with cardiovascular diseases. Cardiac deaths and nonfatal myocardial infarction (MI) occur in about 0.2% of all cases of general anesthesia and surgery (about 500,000 events annually). Cardiac deaths account for approximately 40% of all perioperative mortality, the same proportion as sepsis, although in many cases the cause of death is multisystem organ failure. These figures underestimate the total effect of cardiovascular diseases because another 500,000 persons a year suffer non-fatal MI, unstable angina, or congestive heart failure (CHF) perioperatively, prolonging both their time in the intensive care unit and the total hospital stay.
Although there is great potential to reduce perioperative cardiovascular risk, it is also impractical, unnecessary, and potentially harmful to perform cardiovascular testing in all patients prior to noncardiac surgery. Therefore, it is important to determine perioperative risk, decide whether cardiac testing is appropriate, and provide prophylactic treatment to reduce risk.
 Preoperative Risk Assessment
Preoperative Risk Assessment
An individual patient’s preoperative risk profile depends on three main factors: the patient’s age, current medical and functional status, and the type of surgery. Preoperative electrocardiography can detect arrhythmias and prior silent MI, but it rarely changes management. Preoperative echocardiography would probably provide more useful information, but the cost effectiveness of such testing has not been determined.
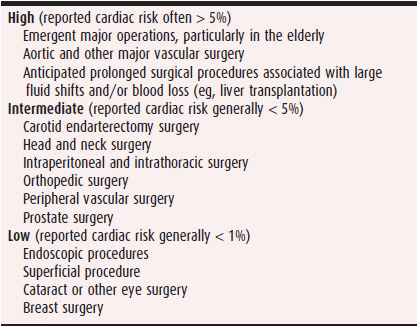
Table 10–1 lists cardiac risk based on type of noncardiac surgery. In the evaluation of perioperative patients, understanding the nature of the surgery is of prime importance. Is this an emergency surgery? If yes, the clinician should advise to proceed with the surgery and evaluate the patient’s cardiac risk postoperatively. On the other hand, if the patient is young, without systemic disease, and undergoing a minor surgery or procedure, the clinician should advise to proceed with surgery without further cardiac workup. However, most patients who require perioperative cardiac consultation are not so straightforward. In these patients, there are various algorithms that can help identify perioperative risk and the need for further cardiac testing.
Table 10–1. Cardiac Risk Stratification for Noncardiac Surgical Procedures
A. Algorithms
1. Revised Cardiac Risk Index (RCRI)—This algorithm is simple to use, and it helps identify patients who require β-blockers perioperatively. However, the RCRI may have less accuracy in patients undergoing major vascular surgery. Use the RCRI as follows:
a. Assign 1 point to each of the following risk factors if present:
• High-risk surgery (intraperitoneal, intrathoracic, suprainguinal vascular)
• Ischemic heart disease (history of MI or current angina, use of sublingual nitroglycerin, recent abnormal stress test, Q waves on electrocardiogram [ECG], or history of coronary revascularization with ongoing chest pain)
• History of heart failure
• History of cerebrovascular disease (stroke, transient ischemic attack)
• Diabetes mellitus requiring insulin
• Preoperative creatinine > 2.0 mg/dL
b. Assign a risk class to determine the major cardiac complication rate to help counsel the patient and also the surgeon:
• Class I: zero risk factors, 0.4%
• Class II: one risk factor, 0.9%
• Class III: two risk factors, 6.6%
• Class IV: three or more risk factors, 11.0%
c. Patients who are categorized as having risk class III or IV may require additional cardiac testing for risk stratification and more aggressive perioperative medical management to reduce the risk of complications.
2. American College of Cardiology/American Heart Association (ACC/AHA) guidelines—These guidelines are somewhat cumbersome. In simplified terms, the ACC/AHA guidelines recommend noninvasive cardiac stress testing in patients with two or more of the following risk factors:
• Intermediate clinical predictors: mild angina, prior MI, compensated or prior heart failure, diabetes mellitus, or renal insufficiency
• Poor functional capacity (< 4 metabolic equivalents): cannot walk more than one or two blocks on level ground; cannot do light housework, such as washing dishes or dusting; cannot climb a flight of stairs or walk up a hill
• High-risk surgery: vascular surgery, prolonged procedure, or anticipated large fluid shifts or blood loss
3. Online cardiac risk calculator—With the increased use of mobile computerized devices by physicians, access to interactive surgical databases is possible. This has been used successfully by cardiac surgeons to assess the risk of cardiac surgery by the use of the Society of Thoracic Surgery (STS) database and the Euroscore from the European Society of Cardiology database. These societies collect data from their members about each surgery performed and its outcome. Then they use multiple regression analysis of the clinical variables in the database to compare with a specific patient’s same variables entered into a Web-based calculator to determine the risk of a proposed surgery. The beauty of these databases is that they are constantly being updated, so as surgical techniques and medical therapy improve these advances are reflected in the risk calculation. A similar schema has been proposed and tested for noncardiac surgery by a group of investigators in Canada and has been shown to be superior to the RCRI. It is based on five variables: the type of surgery; the functional status of the patient; serum creatinine; American Society of Anesthesiology class; and age. We predict that such online Web-based approaches will be the dominant approach to preoperative risk stratification for noncardiac surgery in the future.
B. Intermediate-Risk Patients
Although perioperative management of low-risk and high-risk patients is relatively straightforward, the management of patients who fall into the intermediate-risk group is more challenging. Low-risk patients can proceed to surgery without further cardiac evaluation. For high-risk patients, management should include one or more of the following: postpone or cancel surgery until high-risk features improve or resolve, start treatment of the underlying high-risk features, or proceed to invasive testing. In high-risk patients with a high pretest probability of disease, noninvasive tests are not helpful, because a negative result will most likely be a false negative.
Intermediate-risk patients derive the most benefit from perioperative medical management or stress testing, or both.
For most patients in the intermediate-risk category, it is important to obtain imaging with the stress test because ECG alone is unlikely to move the posttest probability beyond the threshold for treatment or no treatment.
1. Exercise versus pharmacologic stress testing—The choice between exercise and pharmacologic stress testing follows the same guidelines as those for routine, nonperioperative stress testing. Exercise testing can provide valuable information on functional capacity, but patients may not be able to reach 85% of maximal predicted heart rate due to deconditioning or β-blocker use. Since β-blockers are important in the perioperative period, withholding them is not ideal.
Dipyridamole or adenosine is preferred in cases where arrhythmia (eg, rapid atrial fibrillation) or frequent premature atrial or ventricular beats are present. These agents are relatively contraindicated in patients with significant bronchospasm, in whom dobutamine is the agent of choice.
2. Type of imaging technique—When choosing either echocardiography or nuclear imaging (the two most commonly available stress imaging modalities), it is most important to determine which modality has better reliability and expertise at the clinician’s hospital or clinic. In published studies, both imaging techniques have good negative predictive value (> 90%) but poor positive predictive value (< 25%). Echocardiography with contrast (to assist in endocardial border definition) may be more helpful in obese patients (who may have more attenuation defects on nuclear imaging). Nuclear imaging may be more useful in patients with left bundle branch block and when atrial fibrillation is present. Newer modalities include rubidium positron emission tomography (PET) scanning, which provides better resolution images. However, patients must be cooperative and must be able to hold still for longer periods of time. Cardiac computed tomography provides anatomic assessment of the coronary arteries but does not provide data on ischemic burden and has not been adequately evaluated in the perioperative setting.
C. Understanding Cardiac Complications
Despite the risks during general anesthesia, including myocar-dial depression, transient hypotension, and tachycardia, very few cardiac events occur during the surgery itself. The incidence of perioperative cardiac complications actually peaks between 2 and 5 days postoperatively. These data imply that factors activated during or following surgery, and not only the surgery itself, are crucial in determining adverse outcomes.
Pneumonitis and microatelectasis produce ventilation-perfusion mismatch, and sedation or analgesia may cause respiratory depression and interfere with coughing, all of which contribute to arterial hypoxemia. Thrombocytosis and a generalized hypercoagulable state, caused by increased fibrinogen and activators from the damaged tissue, favor thrombosis. At the same time, sympathetically mediated increases in heart rate, blood pressure, and contractility increase myocardial oxygen consumption, whereas thrombotic tendencies, anemia, and arterial desaturation impede oxygen delivery to the myocardium. In a patient with underlying coronary artery disease, this situation may lead to myocardial ischemia or infarction. The imbalance may be further exaggerated because antihypertensive or anginal medications are often withheld. By the third or fourth postoperative day, the patient is hypermetabolic, with negative nitrogen and potassium balances. A natriuresis follows, which can produce hypovolemia and further activate the sympathetic nervous system.
All of these factors provide the exact setting in which a perioperative MI may occur. Perioperative MI carries a high mortality and presents atypically (usually without chest pain). Clues such as hypotension, pulmonary edema, altered mental status, and arrhythmia may be the only signs alerting the clinician to the possibility of a perioperative MI. Therefore, those caring for perioperative patients must have a high level of suspicion in order to detect perioperative MI.
Fleisher LA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 2007;50:1707–32. [PMID: 17950159]
Gupta PK, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–7. [PMID: 21730309]
 Treatment to Reduce Perioperative Risk
Treatment to Reduce Perioperative Risk
Knowledge of how new therapies can reduce cardiac risk has progressed rapidly in recent years. Perioperative medicine now provides “risk management” (through medicine such as β-blocker) in addition to the standard risk prediction. In general, the higher the patient’s risk, the greater benefit derived from the use of therapies such as β-blockers and statins. Therefore, healthcare providers should risk stratify patients, and then use this to determine whether or not to treat.
A. β-Blockers
These agents are first-line therapy to reduce perioperative morbidity and mortality in high-risk patients. Current guidelines have advocated β-blockers in almost all intermediate- or high-risk patients undergoing noncardiac surgery. However, two recent studies in vascular surgery patients did not show a benefit, and a meta-analysis of seven randomized controlled trials did not conclusively show a benefit with β-blockers. Another recent retrospective review of 600,000 patients showed that β-blocker benefit was greater in higher risk patients. Therefore, the decision to use β-blockers should be individualized. If the patient is already taking a β-blocker for other reasons (eg, coronary artery disease), β-blockers should be continued in the perioperative setting.
In patients deemed to benefit from β-blockers in the perioperative setting, oral metoprolol is started on the day of the perioperative visit. For patients who were taking β-blockers before the surgery, the dose is titrated to a heart rate of 60 bpm. Postoperatively, metoprolol is started at 5–10 mg intravenously every 4–6 hours as needed to keep the heart rate at 60–70 bpm until the patient can take oral medications. Oral β-blockers are then continued for 30 days postoperatively and indefinitely in patients who were previously taking these medications.
B. Statins
One small randomized controlled trial and several observational studies have found a benefit to statin use, with greater benefit in higher risk patients, especially major vascular surgery patients. Therefore, if there are no contraindications, high-risk patients should be taking a statin prior to major noncardiac surgery. Also, patients already on statins should have them continued because there is evidence that stopping them is associated with a higher perioperative MI rate.
C. Clonidine
There is evidence that clonidine (an α2-adrenergic agonist) reduces MI and mortality in high-risk vascular surgery patients. Clonidine’s transdermal delivery system works well in the perioperative setting, but clonidine is not ideal for outpatient management and carries the risk of rebound hypertension. For these reasons, clonidine should not be used routinely, but can be a good choice for in-hospital control of hypertension in the perioperative patient who cannot take oral medications.
D. Calcium Channel Blockers
Verapamil and diltiazem (centrally acting calcium channel blockers) are second-line therapy for reducing perioperative ischemia or arrhythmia, or both. Use in high-risk patients who have a contraindication to β-blockers.
E. Aspirin
Many patients are taking aspirin for known cardiovascular disease or for its prevention. Aspirin has been shown to mildly increase bleeding risk with surgery, but there are few data on how to manage aspirin perioperatively in patients without coronary stents. A few observational studies have shown increased cardiovascular event rates if aspirin is stopped, but there are not clear guidelines on how to manage aspirin. It seems reasonable to continue low-dose aspirin therapy if a small increase in bleeding risk is acceptable. This is often not the case for cerebral or eye surgery, but perfectly acceptable in orthopedic surgery, for example. If a patient is not on aspirin, it does not make sense to start it before surgery no matter what the indication. If aspirin needs to be stopped, it should be stopped 7–10 days prior to surgery.
F. Deep Venous Thrombosis Prophylaxis
Although not a routine part of perioperative cardiac risk assessment and treatment, it is important to check for appropriate perioperative deep venous thrombosis (DVT) prophylaxis since DVT (with resultant pulmonary embolism) can cause significant cardiac instability and death. Low-molecular-weight heparins are being used increasingly in place of unfractionated heparin and appear to be equivalent or, in some cases, superior.
G. Endocarditis Prophylaxis
Recommendations for management of endocarditis prophylaxis have changed dramatically with the publication of the new AHA guidelines. In general, the new AHA guidelines advocate less use of antibiotic prophylaxis because of the lack of evidence of benefit in humans and the fact that transient bacteremia occurs frequently, and there is no evidence that dental and other procedures increase rates of bacteremia more than activities of daily living alone. The AHA guidelines state that only patients at the highest risk for endocarditis should receive antibiotic prophylaxis. These high-risk patients include those with prosthetic cardiac valve, previous infective endocarditis, unrepaired cyanotic congenital heart disease (including palliative shunts and conduits), completely repaired congenital heart defect with prosthetic material or device during the first 6 months after the procedure, repaired congenital heart defects with residual defects at site of prosthetic material that prevent endothelialization, and patients who have undergone heart transplantation in whom significant valvular heart disease develops.
Prophylaxis for dental procedures in the aforementioned patients is only recommended if the procedure involves manipulation of gingival tissue, manipulation of periapical region of teeth, or perforation of the oral mucosa.
Endocarditis prophylaxis is no longer recommended for genitourinary or gastrointestinal procedures.
H. Perioperative Medication Management
Management of outpatient medications in the perioperative period is underappreciated but extremely important for ensuring an optimal patient outcome. Many times, essential medications are discontinued and not restarted before the patient is discharged from the hospital, leading to potentially disastrous outcomes. Other times, the conversion of oral to intravenous (and back to oral) dosing of medications causes under- or overtreatment.
1. Anticoagulation management—Nowhere is perioperative medication management more important than in anticoagulation, since use of anticoagulants can cause increased bleeding intra- and postoperatively. Alternatively, too little anticoagulation can lead to severe morbidity (eg, stroke, MI, and stent thrombosis) and even death.
A. ORAL ANTICOAGULANTS—One of the most common reasons for cardiac consultation (besides assessing peri-operative risk) is to manage oral anticoagulants (OACs) perioperatively. Although the risks of discontinuing OAC therapy 4 days prior to surgery are low, in the few patients in whom thromboembolism develops, the results (stroke, pulmonary embolism, MI, or death) can be devastating. Therefore, in high-risk patients, bridging with unfractionated or intravenous heparin is important and can reduce perioperative risk of thromboembolism. However, use of heparins can increase bleeding. Therefore, it is important to carefully select patients who will need bridging with heparin. The keys to optimal OAC management are to identify the indication for OAC and assess the patient’s risk for thromboembolism. Bridging with heparin is advised in the following situations: atrial fibrillation and rheumatic heart disease; history of thromboembolism; mechanical heart valve; hyper-coagulable state; venous or arterial thromboembolism in prior 3 months; or acute intracardiac thrombus visualized on echocardiogram.
Bridging is advised on a case-by-case basis (weighing risks and benefits) in patients with significant (recurrent) cerebrovascular disease, bioprosthetic valve in mitral position, atrial fibrillation with multiple risk factors for cardiac embolism, and history of venous thromboembolism (> 3 months ago). Bridging is not advised in patients with bio-prosthetic valve in the aortic position, atrial fibrillation without multiple risk factors for cardiac embolism, or a history of one remote (> 6 months ago) venous thromboembolism.
Warfarin should be stopped 4 days prior to surgery if preoperative international normalized ratio (INR) is 2.0–3.0 (with modification of timing if INR is > 2.0 or < 3.0). Newer OACs may need to be held for longer periods of time. In patients who require bridging with a heparin, there is evidence that low-molecular-weight heparin (eg, enoxaparin) is just as effective and safe (if not more so) than unfractionated heparin. Enoxaparin (1 mg/kg twice daily), if not contraindicated, is started 36 hours after the last dose of warfarin and is discontinued 24 hours prior to surgery. On postoperative day 1, warfarin is restarted at the preoperative outpatient dose. At 24 hours postoperatively, the patient is evaluated and enoxaparin is restarted if hemostasis has been achieved. Once INR is in the therapeutic range, enoxaparin can be discontinued. Consult manufacturer’s guidelines for bridging with the newer OACs.
B. ANTIPLATELET THERAPY IN PATIENTS WITH CORONARY STENTS—Controversy exists regarding the optimal management of antiplatelet therapy in patients undergoing noncardiac surgery, especially in patients with drug-eluting stents. It appears that decreased endothelialization of drug-eluting stents predisposes these patients to stent thrombosis for quite some time after stent placement.
In patients who do not have a coronary stent and who are at low risk for perioperative cardiac events, aspirin, clopidogrel, and newer antiplatelet agents should be discontinued 7–10 days prior to noncardiac surgery and resumed when hemostasis is achieved postoperatively.
In patients with coronary stents, especially drug-eluting stents, the risk of stent thrombosis greatly increases when aspirin and other antiplatelet drugs are stopped prematurely. Recent AHA/ACC guidelines state that aspirin and clopidogrel should not be discontinued for at least 1 month after bare metal stent and for at least 12 months after the placement of a drug-eluting stent. With some of the newer drug-eluting stents, discontinuation of dual antiplatelet therapy may be possible at 6 months. If surgery cannot be deferred, aspirin should be continued in the perioperative period, and in extremely high-risk patients, intravenous glycoprotein IIbIIIa inhibitors (which have a shorter half-life than clopidogrel) can be used to try to prevent stent thrombosis (although the increased risk of significant bleeding must also be taken into consideration).
It is now clear that cardiologists must discuss with their patients the need for prolonged dual antiplatelet therapy prior to percutaneous coronary intervention, so that if non-cardiac surgery is possible in the near future, drug-eluting stents should be avoided.
2. Antiarrhythmics—These medications should be continued up to the day of surgery and, if necessary, in the immediate postoperative period (in an intravenous form).
Amiodarone is a common oral antiarrhythmic that needs to be converted to an intravenous format in the perioperative period. Since intravenous bolus of amiodarone can cause hypotension, it is more ideal to add up the total daily oral dose of amiodarone and convert that dose to a prolonged or continuous infusion (eg, instead of giving 200 mg intravenously as a bolus once daily, give 0.15 mg/min intravenously continuously over 24 hours, or give the 200 mg intravenously over 4–6 hours).
Digoxin dose should generally be reduced slightly in the perioperative period, especially in elderly patients or those in whom worsening renal function is to be expected.
3. Nonsteroidal anti-inflammatory drugs (NSAIDs)—
These medications can predispose elderly and other high-risk patients to perioperative renal failure, especially with perioperative dehydration and hypotension. Therefore, NSAIDs should be discontinued at least 3 days prior to surgery and restarted if necessary upon discharge from the hospital.
I. Prophylactic Coronary Revascularization
For patients who are intermediate or high risk and who eventually undergo coronary angiography, there are three possibilities: no significant coronary artery disease, left main or triple-vessel coronary artery disease, and single- or two-vessel coronary artery disease.
1. No significant coronary artery disease—Noncardiac surgery can proceed without further testing, although peri-operative risk reduction with β-blockers and other agents may be important.
2. Left main or triple-vessel coronary artery disease—These patients, who are on the opposite extreme, are very high risk and should undergo prophylactic coronary artery bypass grafting. Alternatively, in institutions with expertise, percutaneous coronary intervention is another possibility.
3. Single- or two-vessel coronary artery disease—In these patients, the decision for prophylactic revascularization is more difficult. However, there are no convincing data that revascularization, especially with surgery, is superior to maximal medical therapy for the prevention of short- and long-term cardiac events. Therefore, in patients found to have single- or two-vessel coronary disease, medical management in the perioperative period, even before high-risk vascular surgery, is just as safe as prophylactic revascularization and does not delay noncardiac surgery. If patients must undergo percutaneous coronary intervention preoperatively, balloon angioplasty alone avoids the necessity to continue dual antiplatelet therapy. If stents are placed, it is extremely important to follow recommendations for perioperative management of dual antiplatelet therapy (see earlier section, Perioperative Medication Management).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


