In the current era, the vast majority of infants born with complete transposition of the great arteries (d-TGA) undergo an arterial switch operation soon after birth. The success of this procedure has obviated the need for the right ventricle (RV) to function as a systemic ventricle. However, the majority of patients operated on before 1985 underwent other procedures that resulted in a lifelong commitment of the RV to function as the systemic pump. It is important for the echocardiographer to appreciate the nuances of these techniques when evaluating long-term outcome of these patients. This chapter will be devoted to the evaluation of the adult with d-TGA after an atrial switch (Mustard or Senning) and will address some of the long-term issues associated with the arterial switch operation (ASO). The long-term results for the ASO have been excellent. The first generation of adult survivors of the ASO have thrived with minimal impact on their lifestyles. However, this group of patients may encounter unique problems related to progressive neoaortic valve regurgitation and neoaortic root dilation. Echocardiography is uniquely suited to evaluate these issues after an ASO.
HISTORICAL PERSPECTIVE
The evolution of the surgical treatment of complete d-TGA is an important and fascinating story of humans’ ingenuity in dealing with a lethal congenital anomaly. Although survival of patients with d-TGA beyond infancy if associated with atrial (ASDs) and/or ventricular septal defects (VSDs) was possible without operation, the majority of patients with d-TGA died in infancy. Those rare infants who survived did so with significant exercise limitation and the morbidity and mortality associated with erythrocythemia and Eisenmenger syndrome. The earliest operation described for d-TGA was the creation of an ASD by Blalock and Hanlon in 1950. This ingenious operation preceded the introduction of the heart-lung machine and was performed on the beating heart. This operation had a relatively high mortality because these infants were very ill and usually quite acidotic. However, it resulted in survival beyond infancy. In 1956, Baffes described a partial repair of d-TGA. The “Baffes” procedure involved connecting the inferior vena cava (IVC) to the left atrium and connecting the right pulmonary veins to the right atrium. Subsequently, Senning (1959) described rerouting of the system and pulmonary venous return that resulted in complete separation of the pulmonary and systemic venous returns. This was accomplished by using right atrial and atrial septal tissue to construct a baffle. In 1964, in a single case report, Mustard described a similar operation using synthetic material that also resulted in complete separation of the pulmonary and system venous returns. However, both atrial switch operations resulted in the RV remaining as the systemic ventricle. Although the “Mustard” operation had the same end result as the “Senning” operation, the former was more popular in the United States. From 1959 to 1975, the Senning and Mustard operations were the mainstay of “physiologic correction” operations for patients with d-TGA and an intact ventricular septum.
In 1975, Jatene and colleagues resurrected the concept of anatomic repair of d-TGA and described a number of successful arterial switch procedures. Since the early 1980s, the arterial switch operation has been the operation of choice for patients with d-TGA with or without VSD but without significant pulmonary valve stenosis. In the current era, the Senning and Mustard operations are rarely performed, usually only for patients who are not candidates for an arterial switch or Rastelli procedure. In the late 1970s and early 1980s, the operative mortality for the Senning and Mustard operations actually was lower than that for the Jatene procedure. However, it was anticipated that the operative mortality for the Jatene procedure would decline significantly as surgeons gained experience with the operation and that the long-term results for the Jatene operation would be superior to those of the Senning and Mustard operations. Time has proved that both of these assumptions are true.
The quest for an operation to replace the Senning or Mustard procedure was spurred on because of the long-term complications inherent to these operations. The complications included superior vena cava (SVC) or IVC obstruction (reported in 15% of patients), pulmonary vein obstruction (reported in 5% of patients), arrhythmias, and right ventricular (systemic ventricle) failure. Systemic ventricular failure and some serious arrhythmias may be amenable only to cardiac transplantation or, very rarely, conversion of the Mustard/Senning to an arterial switch procedure. Cardiac transplantation is costly and is associated with significant mid- and long-term morbidity and mortality. Conversion to an arterial switch usually requires banding of the pulmonary artery to prepare the left ventricle (LV). Both the pulmonary banding procedure and the arterial switch operation in these adult patients are associated with significant mortality and the long-term outcome is poorly understood. The late arterial switch has generally been abandoned in adult patients.
Patients who had an atrial switch operation (Senning or Mustard) operation for d-TGA encounter unique problems as they enter adulthood. Overall, approximately 80% are alive and generally doing well at 20 years postprocedure. Problems in adulthood include atrial arrhythmia (present in greater than 50%), systemic and pulmonary venous baffle obstruction, and deterioration of the systemic RV. Systemic baffle obstruction is more common after the Mustard operation (SVC is more common than IVC). Pulmonary venous baffle obstruction is more common after the Senning operation. These problems can usually be managed in the cardiac catheterization laboratory with intravascular stent placement or during hybrid minimally invasive surgical/intravascular stent placement procedures. Functional assessment of the systemic RV is challenging and fraught with measurement pitfalls and undependable geometric assumptions. Historically, clinicians have depended on a visual “gestalt” of right ventricular systolic function rather than rigorous quantitation of ejection fraction. Newer techniques to assess function may offer previously unavailable quantitation of systemic RV function.
ASSESSMENT OF THE ADULT AFTER ATRIAL SWITCH OPERATION
After atrial switch operation, not all portions of the systemic venous and pulmonary venous pathways can be visualized in one acoustic window. Standard subcostal, parasternal, apical, and suprasternal windows are used to evaluate these pathways after the Mustard or Senning operation. However, since the vast majority of these patients are currently adults, the subcostal window may have limited utility. Gross visual assessment of right ventricular systolic function should be performed from as many of these windows as available in a given patient. Parasternal long- and short-axis images will typically demonstrate a flattened ventricular septum and a “D”-shaped LV, since the RV is the systemic pump (Fig. 39.1 A,B). The tricuspid regurgitation spectral Doppler signal in these patients represents systemic ventricular pressure and does not indicate pulmonary hypertension. In the apical projection, one can assess the pulmonary venous pathway. This pathway is divided by the SVC systemic venous baffle into a posterior chamber that receives the pulmonary veins and an anterior “right atrium” chamber that contains the inlet of the tricuspid valve. The term “pulmonary venous atrium” is used to describe one or both of these chambers after atrial switch operation. The anatomic connection of the pulmonary veins to the native left atrium is not disturbed during an atrial switch procedure. The flow from the pulmonary veins travels anteriorly and laterally over the SVC baffle to access the tricuspid valve. The complexity of the course of the systemic venous baffles and their relationship to the pulmonary venous flow explains why multiple acoustic windows are needed to fully assess these patients.

Figure 39.1. Adult patient after Mustard operation. A: Parasternal long-axis image demonstrating leftward displacement of the ventricular septum due to the systemic right ventricular (RV) dilation. B: Parasternal short-axis image in the same patient demonstrating a “D”-shaped left ventricle (LV) as a result of septal flattening caused by the systemic systolic pressure in the right ventricle. C: Apical four-chamber image demonstrating RV dilation in this patient. Varying severity of RV systolic dysfunction is present in these adult patients. D: Mild tricuspid regurgitation in the same patient. Progressive tricuspid regurgitation may become a problem as RV systolic dysfunction progresses.
The apical four-chamber window provides an excellent assessment of RV dilation and tricuspid regurgitation (Fig. 39.1C,D). In addition, the pulmonary venous and systemic venous baffles can be assessed from this window. If one begins the scan tilted slightly posterior, the pulmonary venous pathway is readily visualized in most patients (Fig. 39.2). Tilting just anteriorly from the pulmonary venous baffle (returning to a true four-chamber view), so that the mitral valve leaflets are visualized, permits assessment of the IVC portion of the systemic venous baffle (Fig. 39.3). Remembering that the apical four-chamber image is oriented in a posterior plane from superior to apical, the IVC baffle is visualized when the mitral leaflets are seen. Conversely, if one rotates clockwise and tilts slightly anterior into an LV outflow tract view, then sometimes the SVC portion of the systemic venous baffle may come into view. The SVC baffle is best evaluated from the parasternal long-axis projection with medial tilt of the transducer (Fig. 39.4). The portion of the pulmonary venous pathway that is posterior to the SVC baffle can also be evaluated in this imaging plane. If an SVC baffle obstruction is present, the suprasternal notch or high right parasternal views may be helpful to obtain a gradient.
Echocardiography plays an important role in the evaluation of systemic and pulmonary venous baffle obstructions in patients after the atrial switch operation. Pulmonary venous pathway obstruction can be detected from the apical four-chamber window (Fig. 39.5). Figure 39.6 demonstrates an adult who had successful resolution of IVC baffle obstruction with placement of an intravascular stent. The IVC baffle stent is well visualized in the apical four-chamber projection (see Fig. 39.6 A,B). Conversely, relieving pulmonary venous baffle obstruction is technically very challenging in the interventional cardiac catheterization laboratory. Either a transseptal or retrograde aortic approach is needed to access the area of pulmonary venous pathway stenosis. Frequently, stent placement in this region is quite difficult due to the circuitous course of the delivery sheath. Newer hybrid techniques using minimally invasive surgical approaches offer much promise for these patients (Figs. 39.7 and 39.8).
Residual atrial level shunts occur rarely in atrial switch patients and these shunts are usually small. Color Doppler assessment of the pulmonary and systemic venous pathways can demonstrate these shunts. However, since no true atrial septum is present in these patients, the residual shunts, if small, may not be readily apparent with surface echocardiography. Dynamic left ventricular (pulmonary ventricle) outflow obstruction may occur in postoperative atrial switch patients. The obstruction is frequently due to subvalvular fibromuscular obstruction, but valvular pulmonary stenosis also occurs. The morphologic LV is designed inherently to be a high-pressure pump and usually tolerates the left ventricular outflow tract (LVOT) obstruction without significant consequence. Any gradient across the LVOT can usually be evaluated from the apical window when one rotates clockwise into an outflow projection. A small portion of patients who had a late (after 1 year of age) atrial switch or an associated VSD may have pulmonary vascular obstructive disease in adulthood. In these patients, the mitral valve regurgitation signal is useful to evaluate pulmonary ventricle systolic pressure. If left ventricular systolic pressure is elevated, one needs to ensure that LVOT or pulmonary venous pathway obstruction is not present.
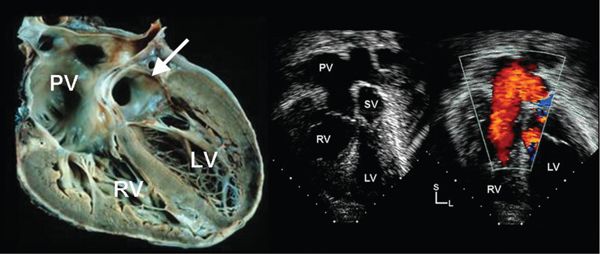
Figure 39.2. Pulmonary venous pathway after atrial switch operation in d-TGA. Left: Pathologic specimen cut to demonstrate the pulmonary venous pathway (PV) after atrial switch operation. A small portion of the systemic venous baffle (arrow) is demonstrated just above the mitral valve. Middle: Apical four-chamber image demonstrating similar anatomy. SV, systemic venous baffle. Right: Color Doppler demonstrates laminar flow from the pulmonary veins to the right atrium. LV, left ventricle; RV, right ventricle.
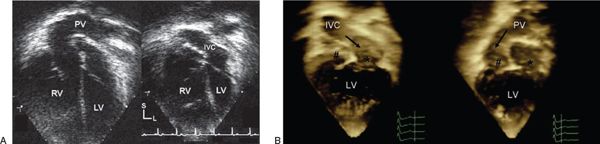
Figure 39.3. Systemic venous pathway (IVC) after atrial switch procedure in d-TGA. A: Tilting into a true apical four-chamber view from the more posterior pulmonary venous pathway (PV) (left), one can now adequately assess the inferior vena cava (IVC) portion of the systemic venous baffle (right). The superior vena cava portion of the systemic venous baffle is not easily visualized in the four-chamber projection. Sometimes the SVC baffle can be partially visualized if one rotates clockwise and tilts anteriorly into the left ventricular outflow tract (LVOT) view. B: Three-dimensional images from the apical four-chamber projection demonstrate the PV (right) and the IVC baffle (left). The relative positions of the right atrium (hash) and left atrium (asterisk) are noted in each panel. LV, left ventricle; RV, right ventricle; IVC, inferior vena cava.

Figure 39.4. Parasternal long-axis image demonstrating the superior vena cava (asterisk) portion of the systemic venous baffle as it enters the left atrium (LA). A portion of the pulmonary venous pathway (PV) is also visualized in this image. LV, left ventricle; RV, right ventricle; PV, pulmonary venous baffle.
NOVEL TECHNIQUES FOR THE FUNCTIONAL ASSESSMENT OF THE SYSTEMIC RIGHT VENTRICLE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


