Chapter 43 Esophagus
History
The earliest record of esophageal disorders dates back to Egyptian times (3000-2500 BC). The Surgical Papyrus, discovered in 1862 by Edwin Smith, described the successful treatment of “a gaping wound of the throat penetrating the gullet.” At the turn of the century, there were significant improvements in anesthesia that allowed for the growth of surgery in many areas, including surgery of the esophagus. In 1901, Dobromysslow performed the first intrathoracic segmental esophageal resection and primary anastomosis, but it was Franz Torek who pioneered the first subtotal esophagogastrectomy in 1913. The use of the stomach to replace the esophagus was first attempted by Leipzin in 1920 and successfully accomplished by Oshava in 1933. A number of modifications occurred over the next 40 years, including changes in approach, anastomosis, and conduits. Ivor Lewis (1946) modified the approach by entering the right chest, and McKewon placed the anastomosis in the neck to eliminate intrathoracic leaks. Although the transhiatal approach had been attempted, it was not well established until 1978, when Orringer and Sloan1 resurrected and perfected this operation, which had been attempted by many before them.
Embryology
Formation of the Gut Tube
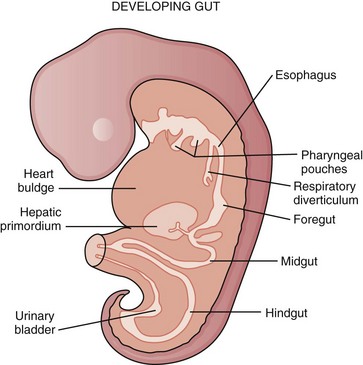
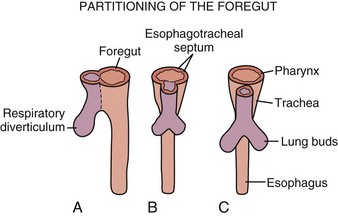
During the embryonic period of development, cephalocaudal (Fig. 43-1) and lateral folding of the embryo occurs. As a result, a portion of the endoderm-lined yolk sac cavity is incorporated into the embryo to form the primitive gut. The primitive gut forms a blind-ending tube consisting of the foregut, midgut, and hindgut (Fig. 43-2). The foregut gives rise to the esophagus. It extends from the pharyngeal tube as far caudally as the liver outgrowth. By the end of week 3 of development, the primitive foregut develops a ventral diverticulum from which the tracheobronchial tree develops. The tracheoesophageal septum gradually partitions this diverticulum from the dorsal portion of the foregut, resulting in a ventral respiratory primordium and dorsal esophagus (Fig. 43-3). During weeks 4 and 5 of development, the rapid growth of the heart and liver allows the esophagus to stretch. As it elongates, the esophageal lumen is almost completely obliterated at the level of the carina. The dorsal esophageal embrace of the trachea results in close approximation of the tracheal bifurcation to the front wall of the esophagus, further narrowing the esophageal lumen.
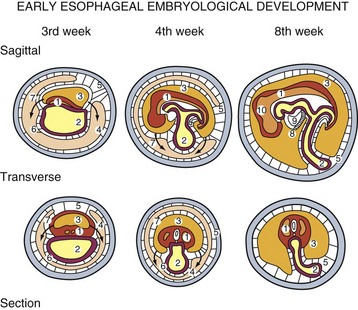
FIGURE 43-1 Early embryologic development.
(Adapted from Pearson FG, Cooper JD, Deslauriers J, et al: Esophageal surgery, ed 2, New York, 2002, Churchill Livingstone, p 20.)
Molecular Regulation of the Gut Tube
Differentiation of various regions of the gut and its derivatives is dependent on a reciprocal interaction between the endoderm (epithelium) of the gut tube and surrounding splanchnic mesoderm. The mesoderm dictates the type of structure that will form, such as the esophagus forming from the foregut, through an HOX code. The induction of the HOX code is a result of sonic hedgehog (SHH) that is expressed throughout the gut endoderm. In the foregut, expression of SHH in the endoderm promotes the expression of the HOX code in the mesoderm. Once specified by this code, the mesoderm instructs the endoderm to form the various components of the foregut.2
Anatomy
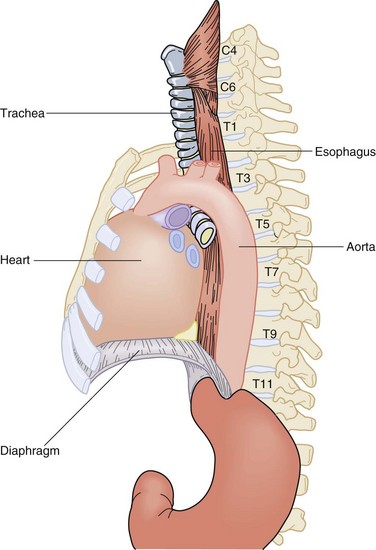
The esophagus is a two-layered, mucosa-lined muscular tube that travels through the neck, chest, and abdomen and rests unobtrusively in the posterior mediastinum. It commences at the base of the pharynx at C6 and terminates in the abdomen, where it joins the cardia of the stomach at T11 (Fig. 43-4). Along its 25- to 30-cm course, it winds its way through a path yielding to structures of more vital efforts. The cervical esophagus begins as a midline structure that deviates slightly to the left of the trachea as it passes through the neck into the thoracic inlet. At the level of the carina, it deviates to the right to accommodate the arch of the aorta. It then winds its way back under the left mainstem bronchus and remains slightly deviated to the left as it enters the diaphragm through the esophageal hiatus at the level of the 11th thoracic vertebra. In the neck and upper thorax, the esophagus is secured between the vertebral column posteriorly and the trachea anteriorly. At the level of the carina, the heart and pericardium lie directly anterior to the thoracic esophagus. Immediately before entering the abdomen, the esophagus is pushed anteriorly by the descending thoracic aorta that accompanies the esophagus through the diaphragm into the abdomen separated by the median arcuate ligament.
Esophageal Layers
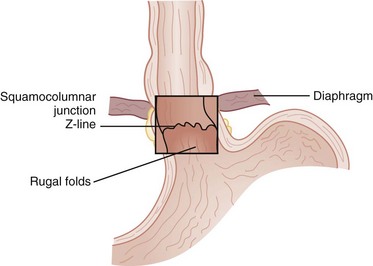
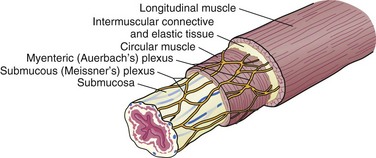
The esophagus is comprised of two proper layers, the mucosa and muscularis propria. It is distinguished from the other layers of the alimentary tract by its lack of a serosa. The mucosa is the innermost layer and consists of squamous epithelium for most of its course. The distal 1 to 2 cm of esophageal mucosa transitions to cardiac mucosa or junctional columnar epithelium at a point known as the Z-line (Fig. 43-5). Within the mucosa, there are four distinct layers—the epithelium, basement membrane, lamina propria, and muscularis mucosae. Deep to the muscularis mucosae lays the submucosa (Fig. 43-6). Within it is a plush network of lymphatic and vascular structures, as well as mucous glands and Meissner’s neural plexus.
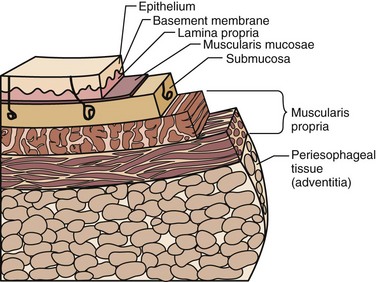
FIGURE 43-6 Layers of the esophagus.
(Adapted from Pearson FG, Cooper JD, Deslauriers J, et al: Esophageal surgery, ed 2, New York, 2002, Churchill Livingstone, p 124.)
Enveloping the mucosa, directly abutting the submucosa, is the muscularis propria. Below the cricopharyngeus muscle, the esophagus is composed of two concentric muscle bundles, an inner circular and outer longitudinal (Fig. 43-7). Both layers of the upper third of the esophagus are striated, whereas the layers of the lower two thirds are smooth muscle. The circular muscles are an extension of the cricopharyngeus muscle and traverse through the thoracic cavity into the abdomen, where they become the middle circular muscles of the lesser curvature of the stomach. The collar of Helvetius marks the transition of the circular muscles of the esophagus to oblique muscles of the stomach at the incisura (cardiac notch). Between the layers of esophageal muscle is a thin septum comprised of connective tissue, blood vessels, and an interconnected network of ganglia known as Auerbach’s plexus. Enshrouding the inner circular layer, the longitudinal muscles of the esophagus begin at the cricoid cartilage and extend into the abdomen, where they join the longitudinal musculature of the cardia of the stomach. The esophagus is then wrapped by a layer of fibroalveolar adventitia.
Gastroesophageal Junction
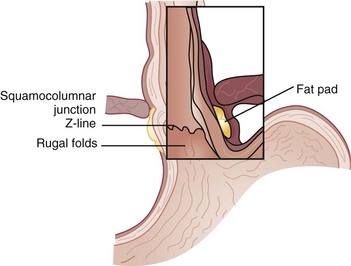
The UES and LES mark the entrance and exit to the esophagus, respectively. These sphincters are defined by a high-pressure zone but can be difficult to identify anatomically. The UES corresponds reliably to the cricopharyngeus muscle, but the LES is more complex to discern. There are four anatomic points that identify the gastroesophageal junction (GEJ), two endoscopic and two external. Endoscopically, there are two anatomic considerations that may be used to identify the GEJ. The squamocolumnar epithelial junction (Z-line) may mark the GEJ provided that the patient does not have a distal esophagus replaced by columnar-lined epithelium, as seen with Barrett’s esophagus. The transition from the smooth esophageal lining to the rugal folds of the stomach may also identify the GEJ accurately. Externally, the collar of Helvetius (or loop of Willis), where the circular muscular fibers of the esophagus join the oblique fibers of the stomach, and the gastroesophageal fat pad are consistent identifiers of the GEJ (Fig. 43-8).
Vasculature
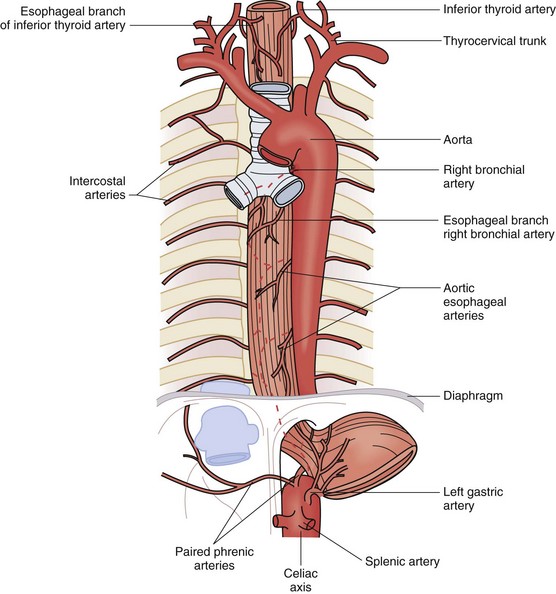
The rich vascular and lymphatic structures that nourish and drain the esophagus serve as a surgical safety net and a highway for metastases. The vasculature is divided into three segments, cervical, thoracic, and abdominal. The cervical esophagus receives most of its blood supply from the inferior thyroid arteries, which branch off of the thyrocervical trunk on the left and the subclavian artery on the right (Fig. 43-9). The cricopharyngeus muscle, which marks the inlet of the esophagus, is supplied by the superior thyroid artery. The thoracic esophagus receives its blood supply directly from four to six esophageal arteries coming off the aorta, as well as esophageal branches off the right and left bronchial arteries. It is supplemented by descending branches off the inferior thyroid arteries, intercostal arteries, and ascending branches of the paired inferior phrenic arteries. The abdominal esophagus receives its blood supply from the left gastric artery and paired inferior phrenic arteries. All the arteries that supply blood to the esophagus terminate in a fine capillary network before they penetrate the muscular wall of the esophagus. After penetrating and supplying the muscular layers, the capillary network continues the length of the esophagus within the submucosal layer.
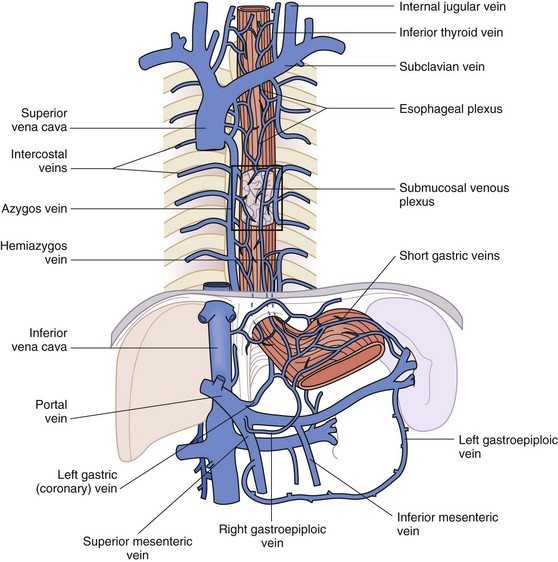
The venous drainage parallels the arterial vasculature and is just as complex. In all parts of the esophagus, the rich submucosal venous plexus is the first basin for venous drainage of the esophagus. In the cervical esophagus, the submucosal venous plexus drains into the inferior thyroid veins, which are tributaries of the left subclavian vein and right brachiocephalic vein (Fig. 43-10). The drainage of the thoracic esophagus is more intricate. The submucosal venous plexus of the thoracic esophagus joins with the more superficial esophageal venous plexus and the venae comitantes that envelop the esophagus at this level. This plexus, in turn, drains into the azygos and hemiazygos veins on the right and left sides of the chest, respectively. The intercostal veins also drain into the azygos venous system. The abdominal esophagus drains into the systemic and portal venous systems through the left and right phrenic veins and left gastric (coronary) vein and short gastric veins, respectively.
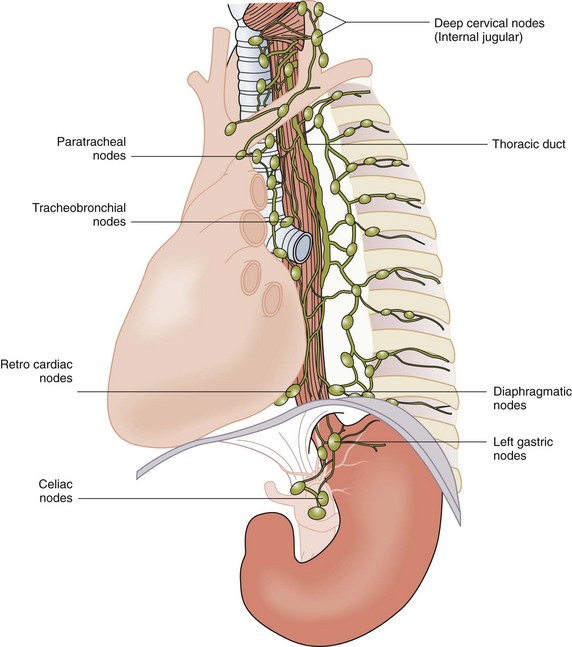
Lymphatics
Other mediastinal stations, such as the para-aortic and inferior pulmonary ligament nodes, can also receive drainage from the thoracic esophagus. Posteriorly, nodes along the esophagus and azygos veins are the primary sites of drainage (Fig. 43-11). The intricate lymphatic network of the esophagus allows for rapid spread of infection and tumor into three body cavities. It stands to reason that the rich arterial supply to the esophagus makes it one of the more durable organs in the body with respect to surgical manipulation, whereas its comprehensive venous and lymphatic drainage create an oncologic challenge to controlling cellular migration. These anatomic complexities lead to surgical challenges when treating esophageal cancer and other esophageal diseases.
Innervation
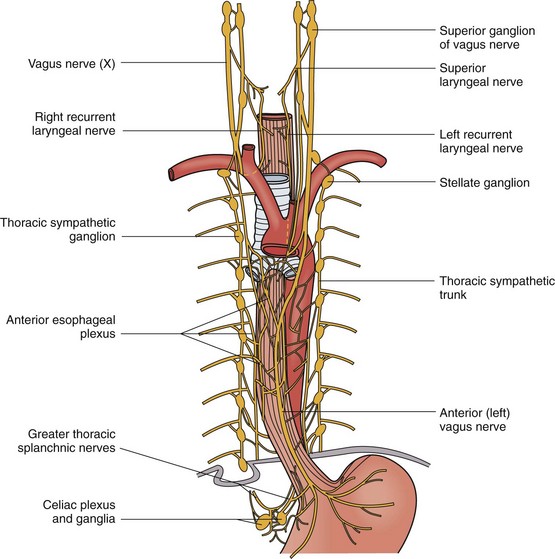
The innervation to the esophagus is sympathetic and parasympathetic (Fig. 43-12). The cervical sympathetic trunk arises from the superior ganglion in the neck. It extends next to the esophagus into the thoracic cavity, where it terminates in the cervicothoracic (stellate) ganglion. Along the way, it gives off branches to the cervical esophagus. The thoracic sympathetic trunk continues on from the stellate ganglion, giving off branches to the esophageal plexus, which envelops the thoracic esophagus anteriorly and posteriorly. Inferiorly, the greater and lesser splanchnic nerves innervate the distal thoracic esophagus. In the abdomen, the sympathetic fibers lay posteriorly alongside the left gastric artery.
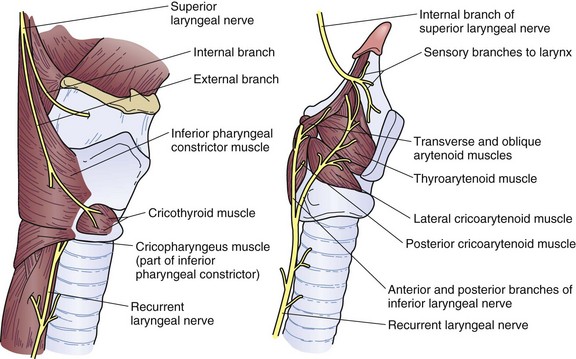
The parasympathetic fibers arise from the vagus nerve, which gives rise to the superior and recurrent laryngeal nerves. The superior laryngeal nerve branches into the external and internal laryngeal nerves that supply motor innervation to the inferior pharyngeal constrictor muscle and cricothyroid muscle and sensory innervation to the larynx, respectively (Fig. 43-13). The right and left recurrent laryngeal nerves come off the vagus nerve and loop underneath the right subclavian artery and aortic arch, respectively. They then travel upward in the tracheoesophageal groove to enter the larynx laterally underneath the inferior pharyngeal constrictor muscle. Along the way, they innervate the cervical esophagus, including the cricopharyngeus muscle. Unilateral injury to the superior or recurrent laryngeal nerve results in hoarseness and aspiration from laryngeal and UES dysfunction. In the thorax, the vagus nerve sends fibers to the striated muscle and parasympathetic preganglionic fibers to the smooth muscle of the esophagus. A weblike nervous plexus envelops the esophagus throughout its thoracic extent. These sympathetic and parasympathetic fibers penetrate through the muscular wall, forming networks between the muscle layers to become Auerbach’s plexus and within the submucosal layer to become Meissner’s plexus (Fig. 43-14). They provide an intrinsic autonomic nervous system within the esophageal wall that is responsible for peristalsis. The parasympathetic fibers coalesce 2 cm above the diaphragm into the left (anterior) and right (posterior) vagus nerves, which descend anteriorly onto the fundus and lesser curvature and posteriorly onto the celiac plexus, respectively.
Physiology
Chicago architect Louis Sullivan is well known for his progressive philosophy that form should follow function. In anatomy this is demonstrated often, and there is no better illustration of this principle in the human body than the esophagus. The primary function of the esophagus is to transport material from the pharynx to the stomach. Secondarily, the esophagus needs to constrain the amount of air that is swallowed and the amount of material that is refluxed. Its form has evolved nicely to enable it to function seamlessly. The esophagus usually measures 30 cm, extending from the pharynx down onto the cardia of the stomach. Under ideal physiologic conditions, the concentric muscular configuration permits effortless unidirectional flow of material from the top to the bottom of the esophagus. The UES, 4 to 5 cm in length, remains in a constant state of tone (mean, 60 mm Hg), preventing a steady flow of air into the esophagus, whereas the tone in the LES (mean, 24 mm Hg) remains elevated just enough to prevent excessive material from refluxing back up into the esophagus (Table 43-1). Transport of a food bolus from the mouth through the esophagus into the stomach begins with swallowing and ends with postrelaxation contraction of the LES, requiring coordinated peristaltic contractions in transit. The material in transit can move easily because the esophageal neuromuscular form provides all functions necessary to power the food bolus through three body cavities.
Table 43-1 Normal Manometric Values
| PARAMETER | VALUE |
|---|---|
| Upper Esophageal Sphincter | |
| Total length | 4.0-5.0 cm |
| Resting pressure | 60.0 mm Hg |
| Relaxation time | 0.58 sec |
| Residual pressure | 0.7-3.7 mm Hg |
| Lower Esophageal Sphincter | |
| Total length | 3-5 cm |
| Abdominal length | 2-4 cm |
| Resting pressure | 6-26 mm Hg |
| Relaxation time | 8.4 sec |
| Residual pressure | 3 mm Hg |
| Esophageal Body Contractions | |
| Amplitude | 40-80 mm Hg |
| Duration | 2.3-3.6 sec |
Swallowing
There are three phases to swallowing, oral, pharyngeal, and esophageal. Six events occur during the oropharyngeal phase of swallowing (Fig. 43-15). These rapid series of events last about 1.5 seconds and, once initiated, are completely reflexive.
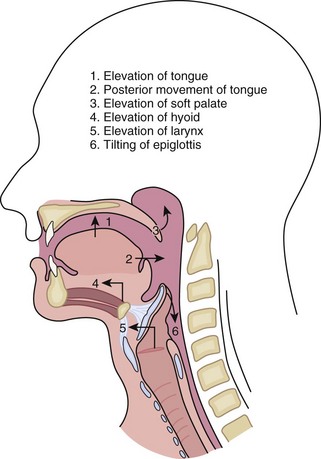
FIGURE 43-15 Phases of oropharyngeal swallowing.
(Adapted from Zuidema GD, Orringer MB: Shackelford’s surgery of the alimentary tract, ed 3, Philadelphia, 1991, WB Saunders, p 95.)
Esophageal Phase
Upper Esophageal Sphincter
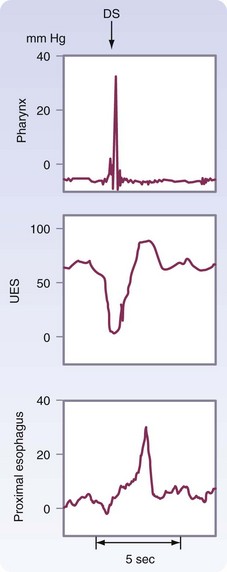
The esophageal phase of swallowing is initiated by the actions during the pharyngeal phase. To allow passage of the food bolus, the UES relaxes and the peristaltic contractions of the posterior pharyngeal constrictors propel the bolus into the esophagus. The pressure differential generated between the positive pressure in the cervical esophagus and the negative intrathoracic pressure sucks the bolus into the thoracic esophagus. Within 0.5 second of the initiation of swallowing, the UES closes, reaching close to 90 mm Hg. This postrelaxation contraction lasts 2 to 5 milliseconds, initiates peristalsis, and prevents reflux of the bolus back into the pharynx. The UES pressure returns to resting pressure (60 mm Hg) as the wave travels into the midesophagus (Fig. 43-16).
Peristalsis
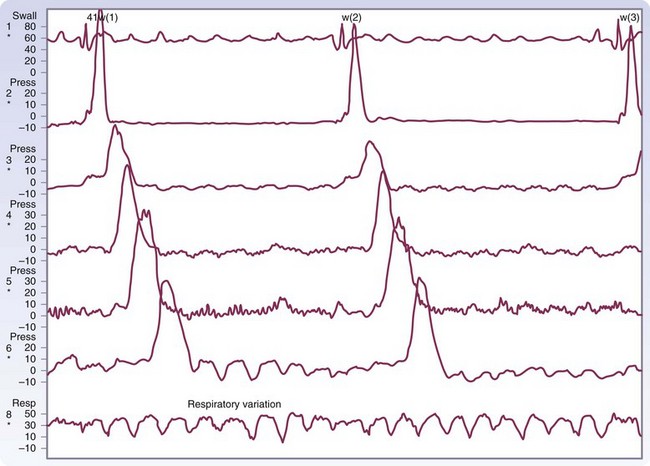
There are three types of esophageal contractions, primary, secondary, and tertiary. Primary peristaltic contractions are progressive and move down the esophagus at a rate of 2 to 4 cm/sec and reach the LES about 9 seconds after the initiation of swallowing (Fig. 43-17). They generate an intraluminal pressure from 40 to 80 mm Hg. Successive swallows will follow with a similar peristaltic wave unless swallowing is repeated rapidly, at which time the esophagus will remain relaxed until the last swallow occurs, and peristalsis will follow. Secondary peristaltic contractions are also progressive but are generated from distention or irritation of the esophagus, rather than voluntary swallowing. They can occur as an independent local reflex to clear the esophagus of material that was left behind after the progression of the primary peristaltic wave. Tertiary contractions are nonprogressive, nonperistaltic, monophasic or multiphasic, simultaneous waves that can occur after voluntary swallowing or spontaneously between swallows throughout the esophagus. They represent uncoordinated contractions of the smooth muscle that are responsible for esophageal spasm.
Lower Esophageal Sphincter
The final phase of esophageal bolus transit occurs through the LES. Although this is not a true sphincter, there is a distinct high-pressure zone that measures 2 to 5 cm in length and generates a resting pressure of 6 to 26 mm Hg. The LES is located in the chest and abdomen. A minimum total length of 2 cm, with at least 1 cm of intra-abdominal length, is required for normal LES function. The transition from the intrathoracic to the intra-abdominal sphincter is noted on a manometric tracing and is known as the respiratory inversion point (RIP; Fig. 43-18). At this point, the pressure of the esophagus changes from negative to positive with inspiration and positive to negative with expiration.
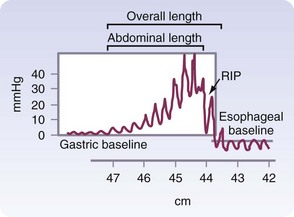
FIGURE 43-18 Normal lower esophageal sphincter.
(From Bremner CG, DeMeester TR, Bremner RM, Mason RJ: Esophageal motility testing made easy, St Louis, 2001, Quality Medical Publishing, p 15.)
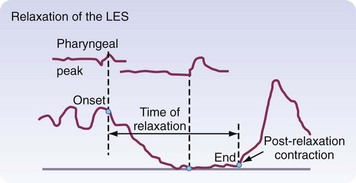
Peristaltic contractions alone do not generate enough force to open up the LES. Vagal-mediated relaxation of the LES occurs 1.5 to 2.5 seconds after pharyngeal swallowing and lasts 4 to 6 seconds. This flawlessly timed relaxation is needed to allow efficient transport of a food bolus out of the esophagus and into the stomach. A postrelaxation contraction of the LES occurs after the peristaltic wave has passed through the esophagus, allowing the LES to return to its baseline pressure (Fig. 43-19), reestablishing a barrier to reflux.
Neuromuscular Disorders of the Esophagus
Diverticula
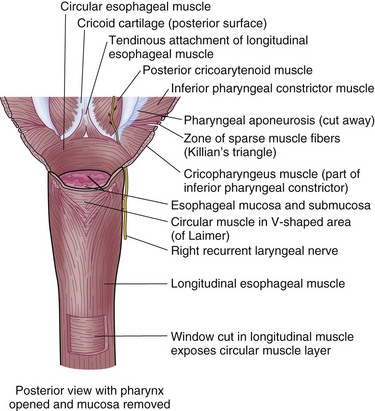
Pharyngoesophageal (Zenker’s) Diverticulum
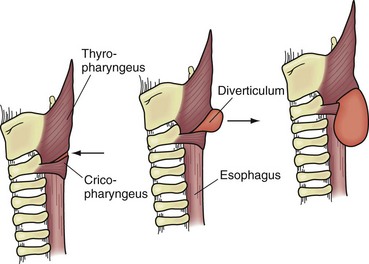
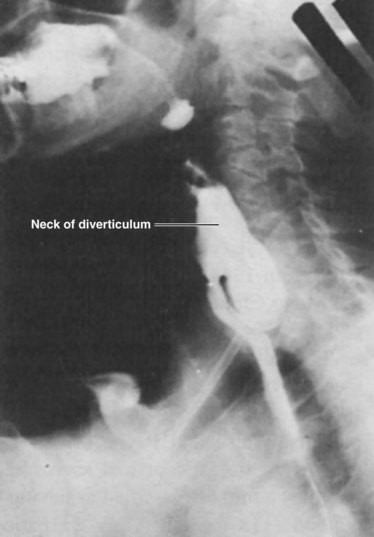
Originally described by Zenker and Von Ziemssen, the pharyngoesophageal diverticulum (Zenker’s diverticulum) is the most common esophageal diverticulum found today. It usually presents in older patients in the seventh decade of life and has been postulated to be a result of loss of tissue elasticity and muscle tone with age. It is specifically found herniating into Killian’s triangle, between the oblique fibers of the thyropharyngeus muscle and the horizontal fibers of the cricopharyngeus muscle (Fig. 43-20). As the diverticulum enlarges, the mucosal and submucosal layers dissect down the left side of the esophagus into the superior mediastinum, posteriorly along the prevertebral space. Zenker’s diverticulum is often referred to as cricopharyngeal achalasia and is managed accordingly.
Symptoms and Diagnosis
Diagnosis is made by barium esophagraphy (Fig. 43-21). At the level of the cricothyroid cartilage, the diverticulum can be seen filled with barium resting posteriorly alongside the esophagus. Lateral views are critical to obtain because this is usually a posterior structure. Neither esophageal manometry nor endoscopy is needed to diagnose Zenker’s diverticulum.
Treatment
Surgical or endoscopic repair of a Zenker’s diverticulum is the gold standard of treatment. Traditionally, an open repair through the left neck was advocated. However, endoscopic exclusion has gained popularity in many centers throughout the United States. Two types of open repair are performed, resection and surgical fixation of the diverticulum. The diverticulectomy and diverticulopexy are performed through an incision in the left neck. Under general anesthesia, they both require about 1 hour to complete. In all cases, a myotomy of the proximal and distal thyropharyngeus and cricopharyngeus muscles is performed. In cases of a small diverticulum (<2 cm), a myotomy alone is often sufficient. In frail patients who may be subject to a higher rate of cervical esophageal leak, a diverticulopexy, without resection, may be performed and will prevent symptoms from recurring.3 In most patients with good tissue or a large sac (>5 cm), excision of the sac is indicated. The postoperative stay is approximately 2 to 3 days, during which the patient remains unable to eat or drink.
The results of open repair versus endoscopic repair have been well studied. For diverticula 3 cm or less in size, surgical repair is superior to endoscopic repair in eliminating symptoms. For any diverticulum larger than 3 cm, the results are the same.4 Both the hospital stay and length of inanition are shorter with an endoscopic procedure. Regardless of the method of repair, patients do well and the results are excellent.
Midesophageal Diverticula
Midesophageal diverticula were first described in the 19th century. Historically, inflamed mediastinal lymph nodes from an infection with tuberculosis accounted for most cases (Fig. 43-22). Infections with histoplasmosis and resultant fibrosing mediastinitis have now become more common. Inflammation of the lymph nodes exerts traction on the wall of the esophagus and leads to the formation of a true diverticulum in the midesophagus. This continues to be an important mechanism for these traction diverticula but it is now believed that some may also be caused by a primary motility disorder, such as achalasia, diffuse esophageal spasm (DES), or nonspecific esophageal motility (NEM) disorder.
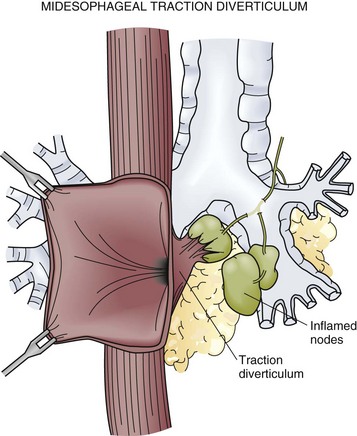
FIGURE 43-22 Midesophageal diverticulum.
(Adapted from Peters JH, DeMeester TR: Esophagus and diaphragmatic hernia. In Schwartz SI, J Fischer JE, Spencer FC, et al [eds]: Principles of surgery, ed 7, New York, 1998, McGraw-Hill, p 1130.)
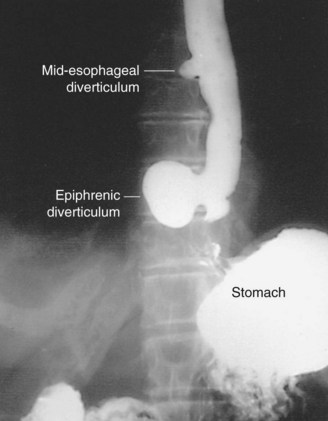
Epiphrenic Diverticula
Symptoms and Diagnosis
A barium esophagram is the best diagnostic tool to detect the presence of an epiphrenic diverticulum (Fig. 43-23). The size, position, and proximity of the diverticulum to the diaphragm can all be clearly delineated. The underlying motility disorder is often identified as well; however, manometric studies need to be undertaken to evaluate the overall motility of the esophageal body and LES. An endoscopy is performed to evaluate for mucosal lesions, including esophagitis, Barrett’s esophagus, and cancer.
Motor Disorders
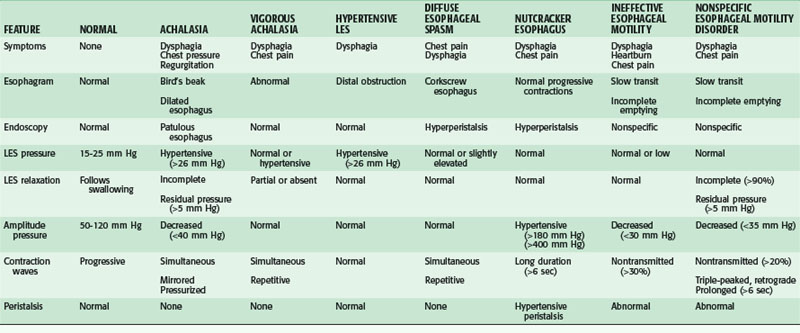
Motility disorders of the esophagus run on a continuum from hypomotile to hypermotile dysfunction, with intermediate types in between. There are primary and secondary motor disorders of the esophagus. Most esophageal motility disorders fall into one of five primary motor disorders: achalasia, DES, nutcracker esophagus, hypertensive LES, and ineffective esophageal motility (IEM; Table 43-2). The use of esophageal manometry has demonstrated a number of nonspecific abnormalities reflecting a spectrum of various stages of destruction of esophageal motor function that do not fit into a specific classification. Secondary motor disorders of the esophagus result from progression of other diseases, such as collagen vascular and neuromuscular diseases, and result in NEM disorders. Although the underlying pathologies are different, the presenting symptoms of primary and secondary motility disorders may be similar. A careful assessment must be done to ensure an accurate diagnosis and appropriate treatment plan.
Achalasia
The literal meaning of the term achalasia is “failure to relax,” which is said of any sphincter that remains in a constant state of tone with periods of relaxation. It is the best understood of all esophageal motility disorders. The incidence is 6/100,000 persons/year and is seen in young women and middle-aged men and women alike. Its pathogenesis is presumed to be idiopathic or infectious neurogenic degeneration.5 Severe emotional stress, trauma, drastic weight reduction, and Chagas’ disease (parasitic infection with Trypanosoma cruzi) have also been implicated. Regardless of the cause, the muscle of the esophagus and LES are affected. Prevailing theories support the model that the destruction of the nerves to the LES is the primary pathology and that degeneration of the neuromuscular function of the body of the esophagus is secondary. This degeneration results in hypertension of the LES and failure of the LES to relax on pharyngeal swallowing, as well as pressurization of the esophagus, esophageal dilation, and resultant loss of progressive peristalsis.
Symptoms and Diagnosis
The diagnosis of achalasia is usually made from an esophagram and a motility study. The findings may vary, depending on the advanced nature of the disease. The esophagram will show a dilated esophagus with a distal narrowing referred to as the classic bird’s beak appearance of the barium-filled esophagus (Fig. 43-24). Sphincter spasm and delayed emptying through the LES, as well as dilation of the esophageal body, are observed. A lack of peristaltic waves in the body and failure of relaxation of the LES are noted. Lack of a gastric air bubble is a common finding on the upright portion of the esophagram and is a result of the tight LES not allowing air to pass easily into the stomach. In the more advanced stage of disease, massive esophageal dilation, tortuosity, and a sigmoidal esophagus (megaesophagus) are seen (Fig. 43-25).
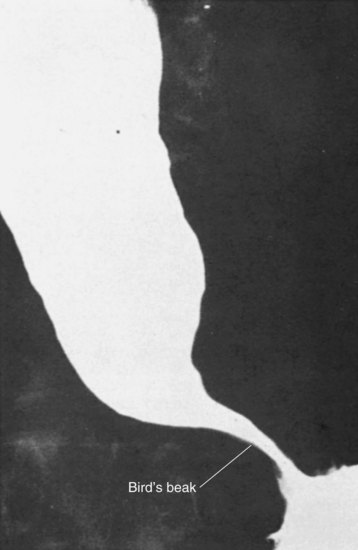
FIGURE 43-24 Barium swallow showing achalasia.
(Adapted from Dalton CB: Esophageal motility disorders. In Pearson FG, Cooper JD, Deslauriers J, et al [eds]: Esophageal surgery, ed 2 New York, 2002, Churchill Livingstone, p 519.)
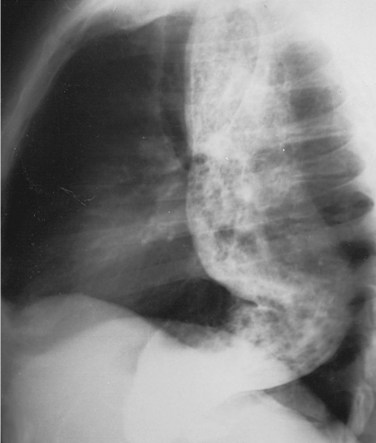
FIGURE 43-25 Barium swallow showing megaesophagus.
(From Orringer MB: Disorders of esophageal motility. In Sabiston DC [ed]: Textbook of Surgery, ed 15, Philadelphia, 1997, WB Saunders, p 719.)
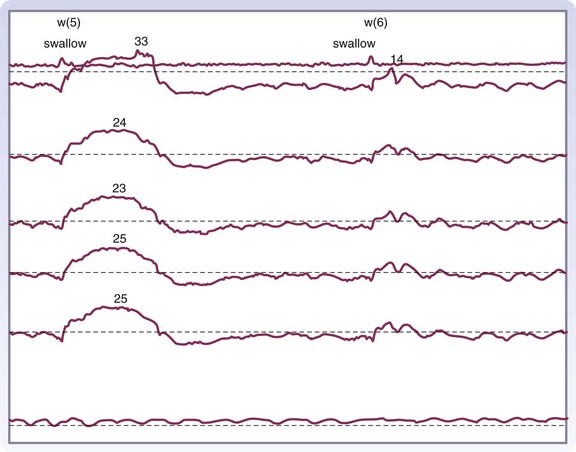
Manometry is the gold standard test for diagnosis and will help eliminate other potential esophageal motility disorders. In typical achalasia, the manometry tracings show five classic findings, two abnormalities of the LES and three of the esophageal body. The LES will be hypertensive, with pressures usually higher than 35 mm Hg but, more importantly, will fail to relax with deglutition (Fig. 43-26). The body of the esophagus will have a pressure above baseline (pressurization of the esophagus) from incomplete air evacuation, simultaneous mirrored contractions with no evidence of progressive peristalsis, and low-amplitude waveforms indicating a lack of muscular tone (Fig. 43-27). These five findings provide a diagnosis of achalasia. An endoscopy is performed to evaluate the mucosa for evidence of esophagitis or cancer. It otherwise contributes little to the diagnosis of achalasia.
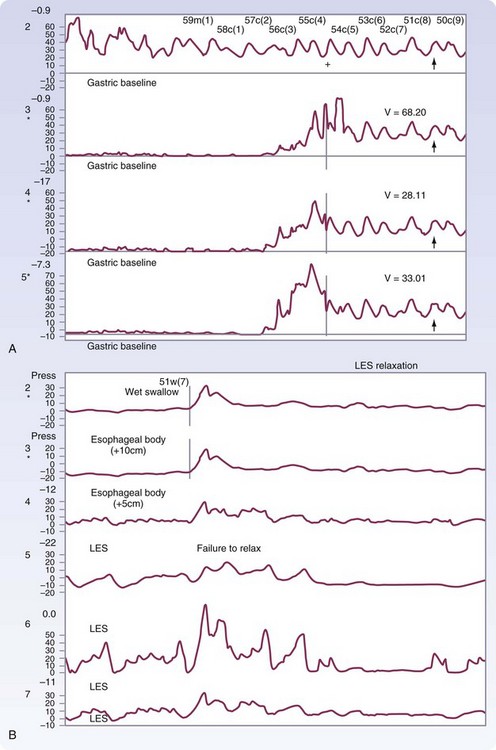
FIGURE 43-26 Motility of the lower esophageal sphincter in a patient with achalasia.
(Adapted from Pearson FG, Cooper JD, Deslauriers J, et al: Esophageal surgery, ed 2, New York, 2002, Churchill Livingstone, p 520.)
Treatment
There are surgical and nonsurgical treatment options for patients with achalasia; all are directed toward relieving the obstruction caused by the LES. Because none of them addresses the issue of decreased motility in the esophageal body, they are all palliative treatments. Nonsurgical treatment options include medications and endoscopic interventions but usually are only a short-term solution to a lifelong problem. In the early stage of the disease, medical treatment with sublingual nitroglycerin, nitrates, or calcium channel blockers may offer hours of relief of chest pressure before or after a meal.6 Bougie dilation up to 54 Fr may offer several months of relief but requires repeated dilations to be sustainable. Injections of botulinum toxin (Botox) directly into the LES blocks acetylcholine release, preventing smooth muscle contraction, and effectively relaxes the LES. With repeated treatments, Botox may offer symptomatic relief for years, but symptoms recur more than 50% of the time within 6 months. Dilation with a Gruntzig-type (volume-limited, pressure control) balloon is effective in 60% of patients and has a risk for perforation less than 4%; however, perforation is life-threatening and must be weighed carefully in otherwise unhealthy patients.
Surgical esophagomyotomy offers superior results and is less traumatic than balloon dilation.7 The current technique is a modification of the Heller myotomy that was described originally by a laparotomy in 1913.8 Various changes have been made to the originally described procedure but the modified laparoscopic Heller myotomy is now the operation of choice. It is done open or with video or robotic assistance. The decision to perform an antireflux procedure remains controversial. Most patients who have undergone a myotomy will experience some symptoms of reflux. The addition of a partial antireflux procedure, such as a Toupet or Dor fundoplication, will restore a barrier to reflux and decrease postoperative symptoms. This is especially true in patients whose esophageal clearance is greatly impaired.9
Esophagectomy is considered in any symptomatic patient with tortuous esophagus (megaesophagus), sigmoid esophagus, failure of more than one myotomy, or an undilatable reflux stricture. Fewer than 60% of patients undergoing repeat myotomy benefit from surgery, and fundoplication for treatment of reflux strictures has even more dismal results. In addition to definitively treating the end-stage achalasia, esophageal resection also eliminates the risk for carcinoma. A transhiatal esophagectomy with10 or without preservation of the vagus nerve offers a good long-term result.
Diffuse Esophageal Spasm
Symptoms and Diagnosis
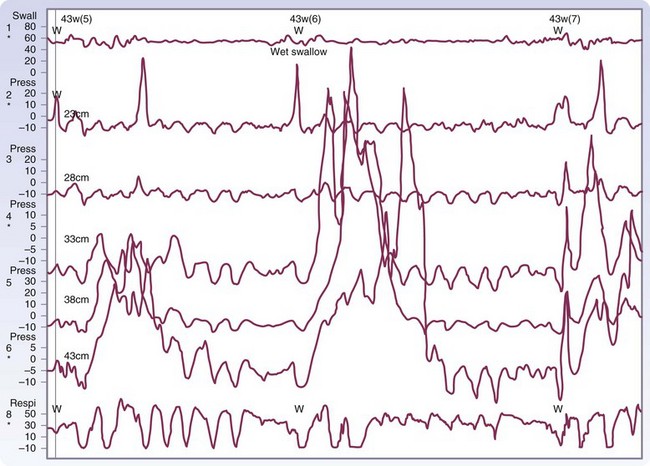
The diagnosis of DES is made by esophagraphy and manometric studies. The classic picture of the corkscrew esophagus or pseudodiverticulosis on an esophagram is caused by the presence of tertiary contractions and indicates advanced disease (Fig. 43-28). A distal bird beak narrowing of the esophagus and normal peristalsis can also be noted. The classic manometry findings in DES are simultaneous multipeaked contractions of high amplitude (>120 mm Hg) or long duration (>2.5 seconds; Fig. 43-29). These erratic contractions occur after more than 10% of wet swallows. Because of the spontaneous contractions and intermittent normal peristalsis, standard manometry may not be enough to identify DES. An ambulatory motility record has been identified as being able to diagnose this disease with a sensitivity of 90% and a specificity of 100% based on an identified set of abnormalities. Correlation of subjective complaints with evidence of spasm (induced by a vagomimetic drug, bethanechol) on manometric tracings is also convincing evidence of this capricious disease.
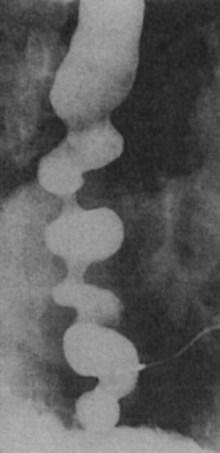
FIGURE 43-28 Barium esophagram of diffuse esophageal spasm.
(Adapted from Peters JH, DeMeester TR: Esophagus and diaphragmatic hernia. In Schwartz SI, J Fischer JE, Spencer FC, et al [eds]: Principles of surgery, ed 7, New York, 1998, McGraw-Hill, p 1129.)
Treatment
The treatment for DES is far from ideal. Today the mainstay of treatment for DES is nonsurgical, and pharmacologic or endoscopic intervention is preferred. Surgery is reserved for patients with recurrent incapacitating episodes of dysphagia and chest pain who do not respond to medical treatment. All patients are evaluated for psychiatric conditions, including depression, psychosomatic complaints, and anxiety. Control of these disorders and reassurance of the esophageal nature of the chest pain that they are experiencing is often therapeutic in and of itself. If dysphagia is a component of a patient’s symptoms, steps must be taken to eliminate trigger foods or drinks from the diet. Similarly, if reflux is a component, acid suppression medications are helpful. Nitrates, calcium channel blockers, sedatives, and anticholinergics may be effective in some cases, but the relative efficacy of these medicines is not known. Peppermint may also provide temporary symptomatic relief.11 Bougie dilation of the esophagus up to 50 or 60 Fr provides relief for severe dysphagia and is 70% to 80% effective. Botulinum toxin injections have also been tried with some success, but the results are not sustainable.
Nutcracker Esophagus
Symptoms and Diagnosis
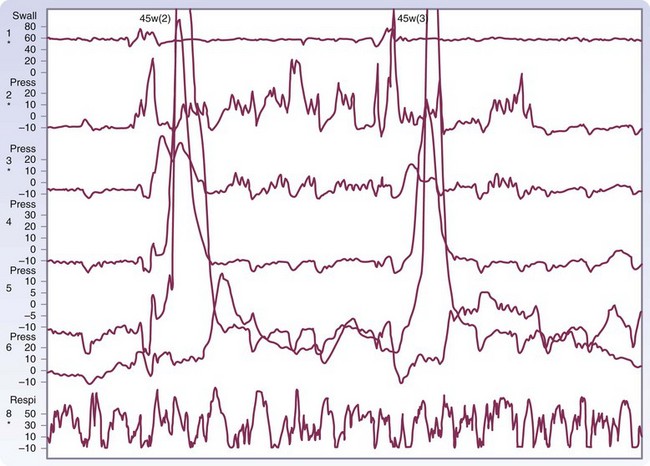
Patients present in a similar fashion to patients with DES with chest pain and dysphagia. Odynophagia is also noted, but regurgitation and reflux are uncommon. An esophagram may or may not reveal any abnormalities. The gold standard of diagnosis is the subjective complaint of chest pain with simultaneous objective evidence of peristaltic esophageal contractions 2 standard deviations (SDs) above the normal values on manometric tracings. Amplitudes higher than 400 mm Hg are common (Fig. 43-30). The LES pressure is normal and relaxation occurs with each wet swallow. Ambulatory monitoring can help distinguish this disorder from DES. This is of critical importance because a subset of DES patients with dysphagia can be helped with esophagomyotomy, but surgery is of questionable value in patients with a nutcracker esophagus.
Hypertensive LES
The condition known as hypertensive LES was first described as a separate entity by Code and colleagues.12 It was observed in patients presenting with dysphagia, chest pain, and manometric findings of an elevated LES. However, the manometric findings are not consistent with achalasia. The LES pressure is above normal and relaxation will be incomplete but may not be consistently abnormal. The motility of the esophageal body may be hyperperistaltic or normal. The pathogenesis is not well understood, but it has been theorized that it may be a similar process to that of achalasia in evolution.
Ineffective Esophageal Motility
IEM was first recognized as a separate disturbance by Castell in 2000.13 It is defined as a contraction abnormality of the distal esophagus and is usually associated with GERD. It may be secondary to inflammatory injury of the esophageal body because of increased exposure to gastric contents. Dampened motility of the esophageal body leads to poor acid clearance in the lower esophagus. Once altered motility is present, the condition appears to be irreversible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree