Esophageal Stents
Wilson S. Tsai
Miguel Alvelo-Rivera
James D. Luketich
The incidence of esophageal cancer has increased dramatically in the past 20 years. Over 15,000 new cases are diagnosed every year, with over 13,000 deaths annually. Esophageal cancer has been an important driving force in the development and improvement of esophageal stents, since a significant proportion of the newly diagnosed cases are not candidates for surgery. Esophageal stents have been used with success in palliating and treating obstructive esophageal diseases. They are most frequently used to treat esophageal malignancies but have also been used to treat benign esophageal pathologies including perforation, anastomotic leaks, and partial dehiscence. By reestablishing esophageal continuity, stents provide patients with rapid relief of dysphagia, a better quality of life, and a chance of relieving malnutrition.
The modern era of esophageal stenting began in the mid-1900s when, in 1959, Celestin first described palliation of an obstructing esophageal cancer with a plastic prosthesis via a laparotomy.5 Atkinson and Ferguson1 later developed endoscopic insertion of a plastic tube, which greatly reduced the complication rate of the procedure.
Initial Patient Evaluation
The initial step in the evaluation of patients for esophageal stent insertion is determining the degree of dysphagia and the ultimate intention of treatment. In most patients, the esophageal stent will be used primarily for palliation from dysphagia while the patient undergoes another form of nonoperative treatment. However, in patients with potentially resectable disease, esophageal stents may relieve symptoms and malnutrition during neoadjuvant therapy, such as chemo- or radiotherapy. In assessing the patient, the physician should carefully evaluate limitations in oral alimentation, the patient’s treatment goals, and his or her performance status. It is also important to understand that the patient’s malnourished state may not be solely due to mechanical obstruction and that systemic catabolism due to metastatic disease may already be established.
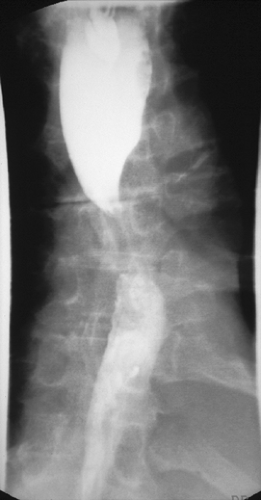
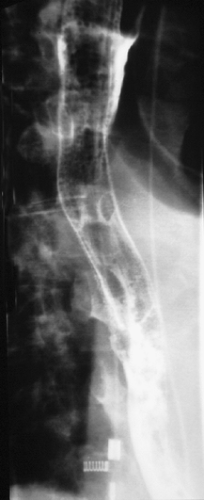
The extent of disease should be confirmed by computed tomography (CT), positron emission tomography (PET), and in some cases endoscopic ultrasound. A barium esophagram should be performed to obtain anatomic specifics on the location, length, and degree of the obstruction while also providing information on other associated abnormalities, such as tracheoesophageal fistulas (Figure 144-1). A flexible esophagogastroscope (EGD) exam is performed to confirm the diagnosis of malignancy, visualize the degree of obstruction in real time, and assess to some degree whether the etiology of obstruction is primarily a fungating endoluminal tumor or extrinsic compression. Finally, in patients with cancers located in the upper two-thirds of the esophagus, a flexible bronchoscopy exam is necessary for tumors involving the middle to upper third of the esophagus to rule out extrinsic or intrinsic involvement of the trachea and left mainstem bronchus. By combining the information from multiple diagnostic tests, the physician can determine which patients will benefit from esophageal stent placement and which will not.
Technique
The authors prefer to place esophageal stents in the inpatient setting. Many patients present with moderate to severe dysphagia and varying degrees of dehydration and malnutrition. While most procedures can be performed under conscious sedation with fluoroscopic guidance, a short general anesthetic with endotracheal intubation minimizes the risk of aspiration and maximizes the chance for optimal stent placement. First, endoscopy is performed to evaluate the location of the pathologic process, the severity of endoluminal obstruction (stricture), the length of the area to be stented, and the condition of the tissues. In the case of a stricture that is too restrictive to allow safe passage of the endoscope, gentle dilation under fluoroscopy is performed. This allows for a complete evaluation with the endoscope to visualize the distal extent of the obstruction and to ensure that the stent is patent distally after its deployment.
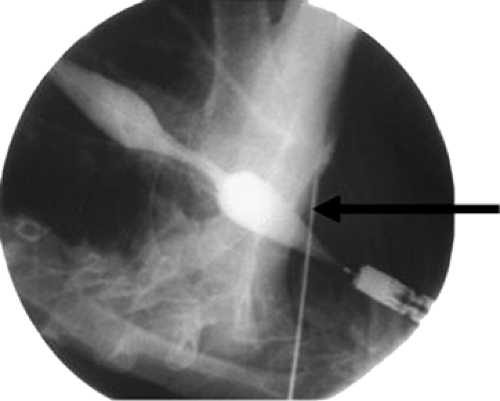
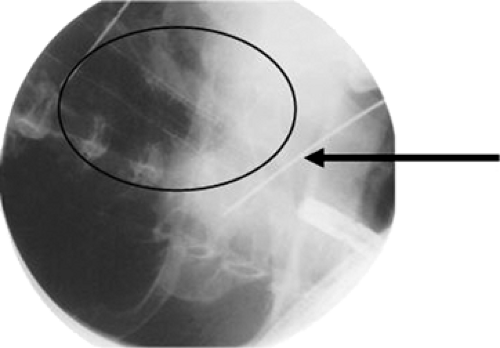
The length of the obstruction is mapped under fluoroscopy with external markers. This is accomplished by aligning two radiopaque markers on the skin with the edge of the scope when it is positioned at the proximal and distal extents of the cancer (Figures 144-2 and 144-3). A guidewire is then passed through the obstruction and the endoscope is withdrawn. The stent length is determined by measuring the distance between the markers and allowing 1 to 2 cm of length beyond the proximal and distal extents. This is to compensate for projection artifact from fluoroscopy. The delivery system (Figures 144-4 and 144-5) is then inserted over the guidewire and, under fluoroscopic guidance, the radiopaque markers on the stent are aligned with the radiopaque markers on the skin. The stent is then slowly deployed. Typically, esophageal stents range from 17 to 23 mm in diameter on maximal expansion following deployment.
Significant pressure on the wall of the stent is preferable after its complete deployment and expansion. Therefore we avoid aggressive dilation of the malignant stricture so as to minimize the risk of complete stent expansion with minimal tension against the tumor and subsequent stent migration. As a final step, we visualize the stent placement endoscopically and fluoroscopically to verify complete expansion of the stent at both the proximal and distal ends, resulting in an hourglass appearance by fluoroscopy (Figure 144-6).
Significant pressure on the wall of the stent is preferable after its complete deployment and expansion. Therefore we avoid aggressive dilation of the malignant stricture so as to minimize the risk of complete stent expansion with minimal tension against the tumor and subsequent stent migration. As a final step, we visualize the stent placement endoscopically and fluoroscopically to verify complete expansion of the stent at both the proximal and distal ends, resulting in an hourglass appearance by fluoroscopy (Figure 144-6).
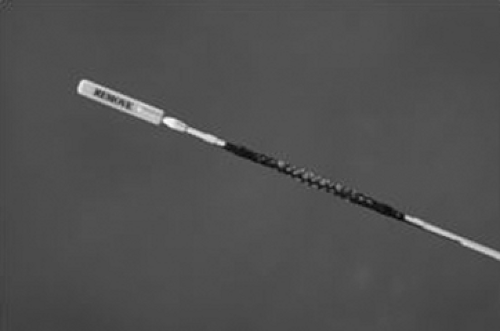
 Figure 144-4. Delivery system of the esophageal stent. (From Perry Y, Luketich JD. The use of esophageal stents. In Cameron JL, ed. Current Surgical Therapy, 8th ed. Philadelphia: Mosby, 2004:49–55.) |
The technical success rate for placement of expandable metal stents ranges from 90% to 100%. Limitations to success include severe pain following placement; tumor extension beyond either the proximal or distal extent of the stent, indicating in some cases a technical error in placement; failure of stent expansion centrally due to too tight a stricture; too large a stent diameter; and immediate stent migration, either proximal or distal. In our experience, severe, procedure-related complications of perforation account for <1% to 2%, with most being immediately addressed by the stent if covered. Aspiration pneumonia requiring hospital admission and intravenous antibiotics has been seen in 1% to 2% of cases. Severe pain requiring stent removal is uncommon (<1% to 2%) and in our experience occurs most commonly in the setting of very large stent diameters (>18 mm). Minor complications, including mild retrosternal pain and gastroesophageal reflux, are experienced by 10% to 20% of patients.16 Delayed complications occur in 30% to 45% of patients and include minor bleeding, fistula formation due to stent erosion into the airway, stent migration, tumor and/or granulation tissue ingrowth or overgrowth, food bolus impaction, and recurrent dysphagia.16
Evolution of Esophageal Stents
Several generations of esophageal stent technologies have been developed, including plastic stents, self-expanding metallic stents (SEMs), and self-expanding plastic stents (SEPs). Esophageal stents were initially manufactured as plastic tubes. However, these stents exhibited a high complication rate (36%), mainly related to perforation and migration, which led to a procedure-related mortality rate of 2% to 16%.10 Furthermore, the internal diameter of these tubes is small (10–12 mm), thereby leading to insufficient dilating effect and continued difficulty in swallowing. An example of a plastic prosthesis is the Celestin Pulsion Tube (Medoc Ltd., Atlanta, GA) which is made of polyethylene. Continued efforts to find a safer method led to the evolution of SEMs in the early 1990s.15,22
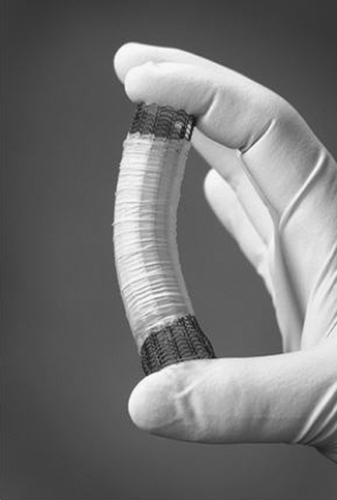
SEMs are available as both noncovered and covered constructions. Noncovered metallic stents for esophageal obstruction were developed first (Figure 144-7). Examples include the Ultraflex and Microvasive Wallstent I (Boston Scientific, Natick, MA). These consist of a metal mesh that expands against the tumor and, by causing localized pressure necrosis, incorporates into the submucosal space of the esophagus. Subsequently, the mesh becomes covered with a fibrinous exudate and is immobilized.40 Although these devices had a lower rate of stent migration, they had the distinct disadvantage of granulation tissue or tumor ingrowth through the metal interstices, leading to a recurrence of the obstruction and dysphagia in 13% to 26% of cases.41 Owing to such experiences with noncovered stents, covered metallic stents were developed in the hope of preventing recurrent obstructions and minimizing the risk of tracheo- esophageal fistula (Figure 144-8).
Silicone, polymer, and polyurethane coverings on the metal struts were developed to prevent tumor ingrowth into the lumen of the stent. A practical disadvantage of this design, however, is a higher rate of stent migration due to lack of integration into the esophageal wall.40 Newer designs include a short segment (usually about 1.5 cm on each end) of noncovered, exposed wire struts to allow proximal and distal integration within the esophageal wall and prevent migration.
However, tumor and tissue growth can still invade through the uncovered portions; therefore obstruction can recur. Granulation tissue overgrowth is another complication in which tissue grows around the edges of the stent, causing difficulties in stent removal or repositioning. Currently, a variety of covered and uncovered metal stents are available: (1) the Ultraflex stent (Microvasive Endoscopy/Boston Scientific Corp., Natick, MA), (2) the Alimaxx-E (Alveolus Inc., Charlotte, NC), (3) the Flamingo Wallstent (Schneider AG, Bulach, Switzerland), (4) the Gianturco-Z stent (Wilson-Cook Europe AIS, Bjaweverskov, Denmark), (5) the Song stent, a modification of the Gianturco Z stent (Sooho Medi-tech, Seoul, Korea), and (6) the EsophaCoil (Medtronic/InStent Inc., Eden Prairie, MN).35 Three of these stents, the Ultraflex, Wallstent, and Gianturco Z stent, have been directly compared in clinical studies. A prospective, randomized study of 100 patients by Siersema et al. in 2001 concluded that all three covered SEMs offered the same degree of palliation for patients with malignant dysphagia. There were differences in the occurrence of major complications among the Gianturco-Z stent (36%), the Ultraflex stent (24%), and the Flamingo Wallstent (18%); however, these differences were not statistically significant. Similar findings of comparable performance among the three SEMs were seen in a study of 150 consecutive patients by Eickhoff et al. However, these authors observed a significantly higher complication rate with the Gianturco-Z stent as compared with the Ultraflex stent and Flamingo Wallstent.9 The Gianturco-Z stent is no longer available in the United States.
However, tumor and tissue growth can still invade through the uncovered portions; therefore obstruction can recur. Granulation tissue overgrowth is another complication in which tissue grows around the edges of the stent, causing difficulties in stent removal or repositioning. Currently, a variety of covered and uncovered metal stents are available: (1) the Ultraflex stent (Microvasive Endoscopy/Boston Scientific Corp., Natick, MA), (2) the Alimaxx-E (Alveolus Inc., Charlotte, NC), (3) the Flamingo Wallstent (Schneider AG, Bulach, Switzerland), (4) the Gianturco-Z stent (Wilson-Cook Europe AIS, Bjaweverskov, Denmark), (5) the Song stent, a modification of the Gianturco Z stent (Sooho Medi-tech, Seoul, Korea), and (6) the EsophaCoil (Medtronic/InStent Inc., Eden Prairie, MN).35 Three of these stents, the Ultraflex, Wallstent, and Gianturco Z stent, have been directly compared in clinical studies. A prospective, randomized study of 100 patients by Siersema et al. in 2001 concluded that all three covered SEMs offered the same degree of palliation for patients with malignant dysphagia. There were differences in the occurrence of major complications among the Gianturco-Z stent (36%), the Ultraflex stent (24%), and the Flamingo Wallstent (18%); however, these differences were not statistically significant. Similar findings of comparable performance among the three SEMs were seen in a study of 150 consecutive patients by Eickhoff et al. However, these authors observed a significantly higher complication rate with the Gianturco-Z stent as compared with the Ultraflex stent and Flamingo Wallstent.9 The Gianturco-Z stent is no longer available in the United States.
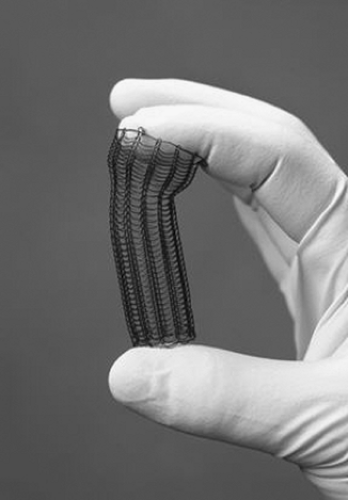 Figure 144-7. Uncovered expanding metallic stent. (From Perry Y, Luketich JD. The use of esophageal stents. In Cameron JL, ed. Current Surgical Therapy, 8th ed. Philadelphia: Mosby, 2004:49–55.) |
Plastic Versus Metal Stents
Since their introduction over a decade ago, SEMs have been favored over the plastic stents for their relative ease of insertion, lower perforation rates, and improved internal diameters (20–25 mm). A retrospective study of 153 patients by Eickhoff et al. showed no significant difference in the total complication rate between a Celestin Pulsion Tube polyethylene prosthesis and various covered SEMs (33% versus 26%, p = 0.456). However, when the complications were further classified as minor or major, a significant difference was shown between the two classes of stents. A multivariate analysis demonstrated a significantly higher rate of major complications in the patients receiving plastic stents over those with SEMs (22% versus 9%, p = 0.05).10 In this study, major complications were defined as bleeding, perforation, stent migration, and stent fracture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree