CHAPTER 38 Esophageal Resection and Replacement
The incidence of esophageal carcinoma, particularly adenocarcinoma, has risen in the Western Hemisphere. The American Cancer Society “Cancer Statistics, 2008” reports that the 5-year survival rate for all patients with esophageal cancer is 15.6%. Patients treated with an esophagectomy have a 5-year survival rate close to 35%.1
HISTORY OF ESOPHAGEAL RESECTION
Ivor Lewis, writing in 1946, is credited with popularizing transthoracic resection of the esophagus. He initially performed the operation in two stages, first mobilizing the stomach through a laparotomy and second, several days later, resecting the esophagus and performing an intrathoracic anastomosis through a right thoracotomy. Lewis showed a sophisticated understanding of the challenges involved in esophageal surgery when he stated that “the oesophagus is a difficult surgical field for three reasons: its inaccessibility; its lack of a serous coat, and its enclosure in structures where infection is especially dangerous and rapid.”2 The Ivor Lewis and transhiatal approaches are the most commonly employed techniques of esophageal resection used today. In 1962, McKeown described a three-stage esophagectomy, with the addition of a cervical incision and anastomosis to allow better margins for proximal tumors.3 It is now called the three-hole or tri-incisional esophagectomy.
Open esophagectomy has up to 60% reported morbidity.4,5 For this reason, several approaches have been supplemented with thoracoscopic or laparoscopic modifications. Since Cuschieri first introduced the concept of a minimally invasive approach to esophagectomy in 1992,6 there have been numerous descriptions in the literature.7–11
TECHNIQUES OF ESOPHAGEAL RESECTION
Modified McKeown or Tri-incisional Technique
Technique
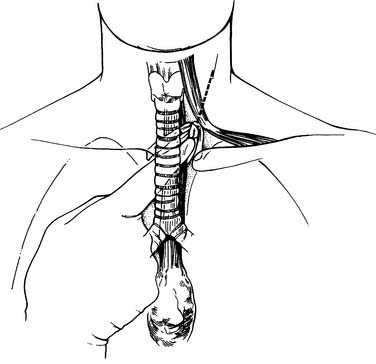
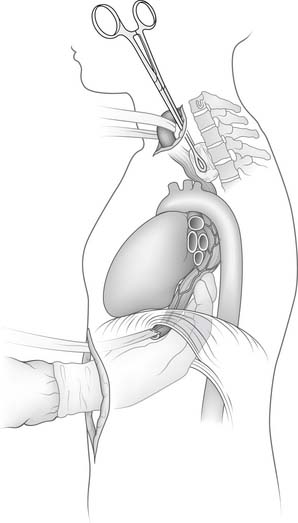
Esophagogastroduodenoscopy is performed to identify the location of the tumor and to rule out disease of the stomach or duodenum. Retroflexion of the esophagogastroduodenoscopy is important for proper evaluation of the gastroesophageal junction. The carina is at the level of 25 cm from the incisor on esophagogastroduodenoscopy. Tumors in this area should be carefully evaluated by bronchoscopy for tumor invasion of the trachea. A double-lumen tube is placed, and the patient is positioned in a left lateral decubitus position. A right posterolateral thoracotomy incision is made wide enough to insert the surgeon’s hand (approximately 10 cm). A portion of the latissimus is divided; the serratus is spared (Fig. 38-1). Division of the intercostal muscle from the vertebral bodies posteriorly to the mammary vessels anteriorly usually provides enough working space without division of a rib. The chest is entered through the fifth or sixth interspace, depending on the location of the tumor.
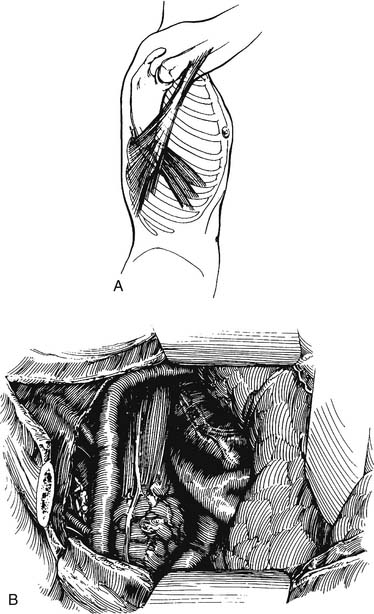
Figure 38–1 A limited right posterolateral thoracotomy is performed, dividing the latissimus.
(From Swanson S, Grondin S, Sugarbaker D. Total esophagectomy: the Brigham and Women’s Hospital approach. Oper Tech Thorac Cardiovasc Surg 1999;4:197-209.)
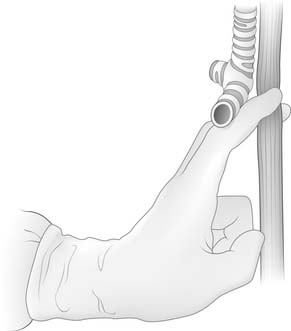
The lung is retracted anteriorly, and the inferior pulmonary ligament is divided by use of cautery. In a region away from the tumor and away from any scarring, the pleura overlying the esophagus is incised anteriorly and posteriorly and the esophagus is surrounded with a Penrose drain. With traction on the Penrose drain, the esophagus is retracted both anteriorly and posteriorly and is dissected by electrocautery, including all adjacent lymph node tissue. Arterial branches supplying the esophagus from the aorta are clipped before being divided. Any cautery performed in the region of the carina must be at low settings to avoid thermal injury to the trachea. The azygos vein is typically divided. At the level of the azygos vein, the vagus nerves are identified on the esophagus and divided between clips. Division of the vagal nerve is important because an intact vagus nerve can lead to a traction injury of the recurrent laryngeal nerve. Further cranial dissection proceeds with the vagus nerves away from the esophagus to avoid recurrent nerve injury. Blunt dissection is useful high in the right chest (Fig. 38-2). Most dissection should be in the posterior plane to avoid injury to the recurrent laryngeal nerve, which is in the groove between the esophagus and trachea. A knotted Penrose drain is placed around the cervical esophagus and pushed into the posterior neck for retrieval during the cervical portion of the case (Fig. 38-3). This Penrose drain, positioned inside the vagus nerves relative to the esophagus, permits isolation of the cervical esophagus without traction on the recurrent nerve. A sponge is packed high in the chest to assist in hemostasis and is removed before closure. The lower portion of the esophagus is encircled with a second Penrose drain and the remaining distal esophagus is dissected, including in the specimen all tissue lateral to the pericardium and medial to the aorta and spine.
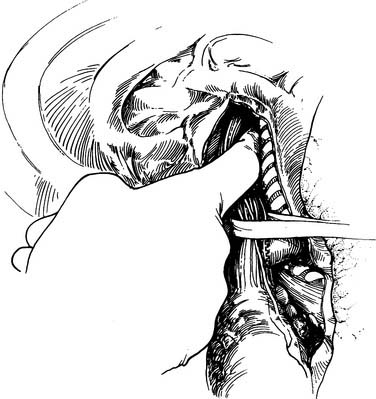
Figure 38–2 Blunt finger dissection close to the esophagus is used to free the esophagus up into the neck.
(From Sugarbaker D, DeCamp M, Liptay M. Surgical procedures to resect and replace the esophagus. In: Zinner M, Schwartz S, Ellis H, et al, editors. Maingot’s abdominal operations. Stanford, CT: Appleton & Lange; 1997. p. 885-910.)
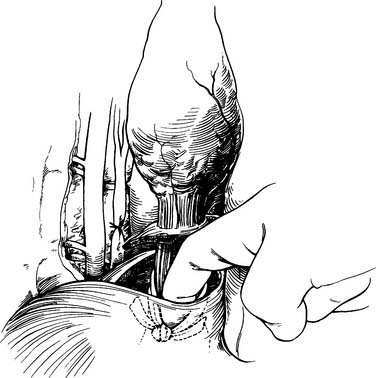
If the tumor is near the gastroesophageal junction, a 2-cm rim of diaphragm is resected with the esophagus (Fig. 38-4). The Penrose drain is knotted and left in the abdomen for retrieval during the abdominal portion of the operation. Mass ligature of the thoracic duct at the level of the hiatus is performed by encircling all tissue between the aorta and spine with a 0 silk suture. The chest is inspected for hemostasis, the sponge previously placed is removed, and a 28 French straight chest tube is inserted through a separate stab incision and directed to the apex of the chest. The ribs are approximated with interrupted #2 Vicryl sutures. The latissimus is closed with running 0 Vicryl suture. The subdermal fascia is closed with a running 2-0 Vicryl suture. The skin is closed with staples or suture. The patient is repositioned in the supine position and is reintubated with a single-lumen tube. A transverse roll is placed under the scapula, and the head is turned 45 degrees to the right. An upper midline laparotomy is performed from the umbilicus to the junction of the costal margin and the left side of the xiphoid; the xiphoid is occasionally resected for better visualization. The abdomen is explored for metastatic disease, which includes inspection of the liver, omentum, and peritoneal surfaces. The left lobe of the liver is mobilized by dividing the triangular ligament. Close to the triangular ligament are large diaphragmatic veins that should be avoided during this dissection. The Penrose drain surrounding the gastroesophageal junction is grasped, and the remaining phrenoesophageal ligament is divided (Fig. 38-5). The gastroepiploic pulse is palpated. At a point 2 cm away from the gastroepiploic artery on the greater curvature of the stomach, the lesser sac is entered. Dissection along the greater curvature of the stomach toward the spleen proceeds with division of vascular branches by use of double clips, silk ties, or the harmonic scalpel. Short gastric vessels are taken in identical fashion. Displacement of the spleen anteriorly by placement of lap pads behind the spleen may aid in dissection of the short gastric vessels. Once the short gastric arteries are divided, additional dissection toward the pylorus is performed. The gastroduodenal artery travels behind the pylorus before branching into the right gastric epiploic and superior pancreaticoduodenal arteries. The duodenum is mobilized from its retroperitoneum connections by a Kocher maneuver. The duodenum is adequately mobilized when the pylorus can reach the esophageal hiatus.
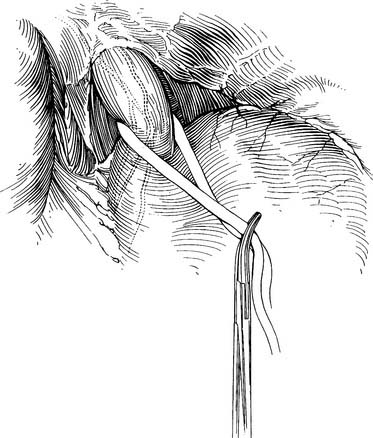
Figure 38–5 The phrenoesophageal ligament is divided fully after retrieval of the Penrose drain.
(From Swanson S, Grondin S, Sugarbaker D. Total esophagectomy: The Brigham and Women’s Hospital approach. Oper Tech Thorac Cardiovasc Surg 1999;4:197-209.)
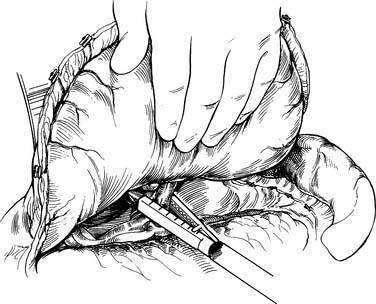
The stomach is then lifted anteriorly, and any adhesions between the stomach and pancreas are divided with electrocautery. The left gastric artery is identified and skeletonized, sweeping lymph node tissue onto the specimen. With a 30-mm vascular stapler, the base of the left gastric artery is clamped. A continued strong pulse in the gastroepiploic artery is confirmed, and the left gastric artery is divided (Fig. 38-6). The gastrohepatic ligament is divided by a combination of cautery and the stapler. Either a pyloromyotomy or pyloroplasty may be performed. If a pyloroplasty is performed, it should be a single layer with interrupted 3-0 silk sutures, carefully incorporating mucosa and muscular wall.
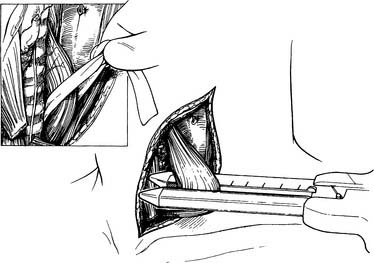
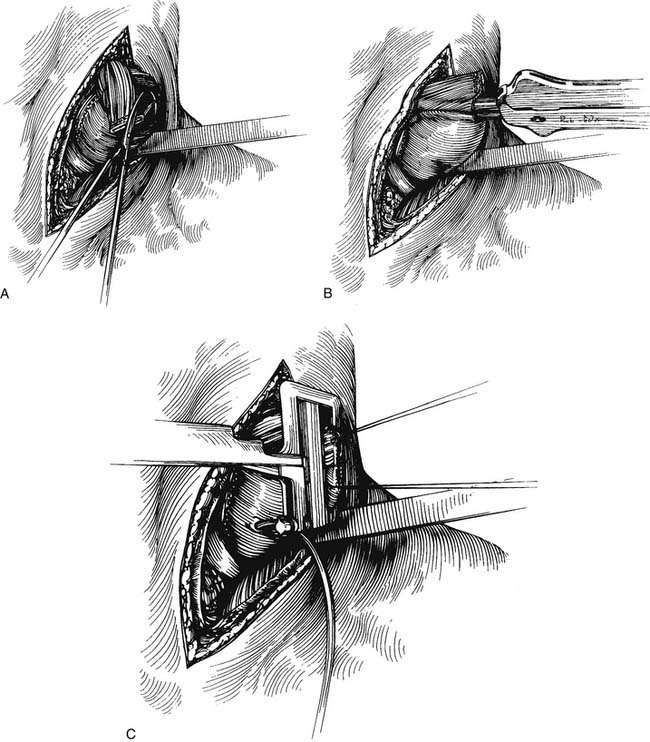
Attention is then turned to the neck, and a 6-cm incision is made from the sternal notch along the anterior border of the sternocleidomastoid muscle. The platysma is divided, and dissection proceeds between the carotid sheath laterally and the strap muscles medially. The omohyoid muscle may be divided. The knot of the Penrose drain should be palpable on the spine. The Penrose drain is grasped, and the esophagus is gently mobilized. The nasogastric tube is partially withdrawn, and the cervical esophagus is divided with a 75-mm linear stapler (Fig. 38-7). A #2 silk suture ligature is fastened to the distal end of the divided esophagus, and the specimen is drawn into the abdomen with the attached silk suture. The cervical end of the silk suture is fastened to a clamp.
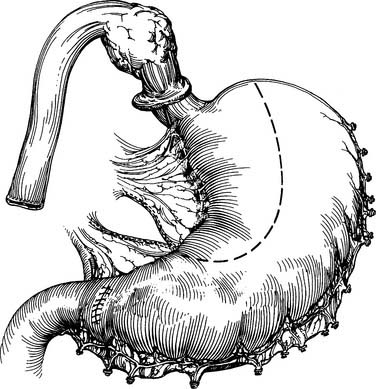
A gastric tube is fashioned by resecting the gastroesophageal junction and the lesser curve of the stomach with a series of 75-mm-thick tissue staples (Fig. 38-8). A narrow gastric tube aids in gastric emptying. A diameter of less than 6 cm, however, can compromise venous and arterial blood supply. The line of division ends at a point on the lesser curve near the crow’s-feet of veins. At this point along the lesser curve, the right gastric artery and associated tissue can be divided to maximize conduit length.
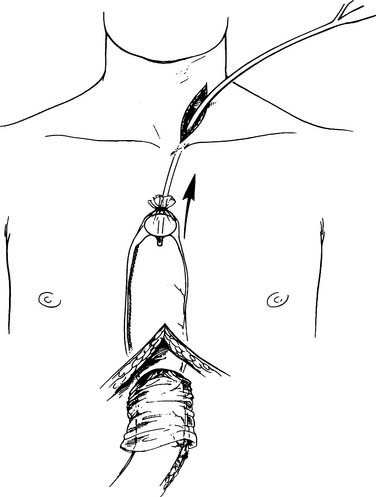
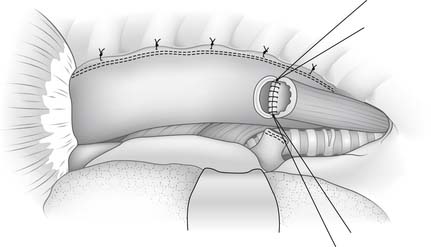
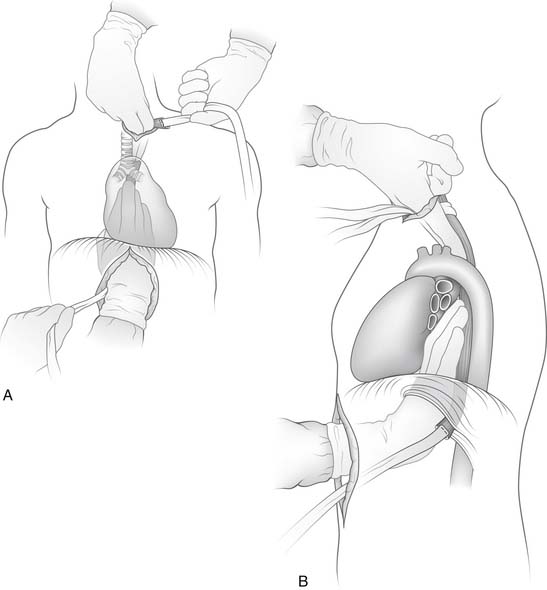
With the specimen removed, a final check for hemostasis is made in the bed of the stomach and spleen. Before the conduit is pulled into the neck, the esophageal hiatus should be dilated to four fingers. The gastric conduit may be pulled to the neck in relatively atraumatic fashion by the use of an endoscopic camera bag attached to a Foley catheter. The heavy silk tie that traverses the mediastinum from the neck is tied to the valved end of a 30-cm3 three-way Foley urinary catheter. The endoscopic bag is secured to the Foley balloon. The conduit is placed in the bag, and the valved end is drawn into the neck (Fig. 38-9). The assistant must guide the conduit through the hiatus and up the lower mediastinum, ensuring that there is no torsion. The bag is cut away from the conduit in the neck, and the conduit is grasped with a Babcock instrument. The pylorus should sit at the hiatus. A side-to-side, functional end-to-end stapled anastomosis may be performed. The anastomosis is created with a 75-mm linear stapler followed by a 30-mm endoscopic stapler. Alternatively, the neck anastomosis can be hand sewn with interrupted circumferential full-thickness 3-0 silk sutures (Fig. 38-10). The nasogastric tube is advanced across the anastomosis and positioned just proximal to the pylorus before closure of the remaining anastomotic defect (Fig. 38-11). A drain is placed along the spine posterior to the anastomosis, and the platysma is run closed with 2-0 Vicryl sutures. The skin is closed with staples. A feeding tube is inserted in the jejunum at a point 40 cm distal to the ligament of Treitz. The abdominal fascia is closed with a running #2 monofilament suture, and the skin is closed with staples.
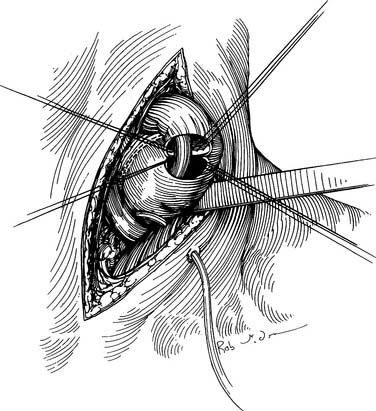
Figure 38–10 The cervical anastomosis may be hand sewn with interrupted silk sutures over a nasogastric tube.
(From Swanson S, Grondin S, Sugarbaker D. Total esophagectomy: The Brigham and Women’s Hospital approach. Oper Tech Thorac Cardiovasc Surg 1999;4:197-209.)
Ivor Lewis Esophagectomy
Technique
With all techniques, wrapping of the anastomosis with omentum and passage of the nasogastric tube before completion of the final portion of the anastomosis are advised. A stapled anastomosis may be performed in a side-to-side, functional end-to-end manner with creation of the anastomosis by a GIA 75-mm or sequential 30-mm endoscopic stapler and closure of the defect with a TA 30-mm or 60-mm stapler. A similar technique involves lining up the gastric conduit and esophagus in a linear fashion and stapling the back wall of the anastomosis with a linear stapler or endostapler and sewing the front wall by either a one- or two-layer technique. This type of approach is used in the neck as described by Orringer. The anastomosis can also be performed with an EEA stapler, although anastomoses created with EEA staplers smaller than 33 mm carry a significant risk of stricture.12 With the EEA stapler, passage of the nasogastric tube is, of course, performed after completion of the anastomosis.
If a hand-sewn anastomosis is to be performed, the proximal esophagus is divided with a knife after clamping the esophagus proximally with a noncrushing bowel clamp. A single-layer anastomosis is performed with interrupted 3-0 silk stitches with full-thickness bites of the mucosa and muscularis and careful approximation of tissues. Knots on the posterior row may be tied inside the lumen (Fig. 38-12).13 A double-layer anastomosis was described by Churchill and Sweet in 1942.14,15 At a point at least 2 cm beyond the staple line, a 2-cm-diameter area of gastric serosa is scored. Underlying vessels are ligated with interrupted silk sutures. The posterior layer of the anastomosis is created with interrupted 3-0 silk stitches. An inner, full-thickness layer is performed with a fine 4-0 absorbable suture, either catgut or Monocryl, by a running Connell stitch to invert the mucosa. Before completion of the inner layer, the nasogastric tube is passed to the hiatus. An anterior outer layer is then performed with interrupted 3-0 silk. Omentum is used to wrap the anastomosis. The distal portion of the stomach is reduced into the abdomen to avoid excess redundancy of the stomach in the chest, which might impair conduit emptying. Stitches are placed anchoring the conduit to the diaphragmatic hiatus. Some use additional stitches anchoring the intrathoracic conduit to the pleura, although the effectiveness of these stitches is debated.
Transhiatal Esophagectomy
Technique
Dissection caudally is performed with two fingers against the esophagus in the posterior plane. Depending on the length of the surgeon’s fingers and the width of the abdominal surgeon’s hand, it should be possible for the two hands to contact directly. There always remains a thin plane of tissue that must be torn for the two hands to touch. Alternatively, a sponge stick can be advanced posteriorly from the neck to touch the dissecting hand from the abdomen (Fig. 38-13). Dissection then proceeds in a similar fashion on the anterior surface of the esophagus. Extreme care must be taken in the region of the trachea and especially the carina to avoid injury to the membranous trachea. (At this point in the operation, any difficulty in dissecting the esophagus from the trachea or any concern of adhesion to or invasion of the trachea should result in repositioning of the patient in the left lateral decubitus position, and a right thoracotomy should be performed for direct visualization and dissection.) The fingers from above and below should meet in the region of the carina (Fig. 38-14).
As much dissection as possible should be performed under direct vision from the neck and from the abdomen. Portions of the lateral dissection near the lower aspect of the trachea often cannot be visualized, and lateral dissection must be performed bluntly. The surgeon’s right hand is advanced from the abdomen high up in the chest to the point where circumferential dissection has been completed from the neck. The first and second fingers surround the esophagus, and with a raking motion, the lateral attachments are avulsed as the hand is drawn back into the abdomen. Care must be taken near the region of the azygos vein (Fig. 38-15). After complete mobilization of the esophagus, it is divided in the neck, and the specimen is brought out into the abdomen. The mediastinum is packed for hemostasis. Before the gastric tube is drawn up into the neck, the mediastinal packing is removed and both pleural spaces are inspected for integrity. Entry into the pleural space is managed by chest tube placement before removal of the drapes.
Trials Comparing Transhiatal with Transthoracic Resection
Numerous nonrandomized, retrospective trials have been performed in an attempt to define differences in either perioperative complication rate or long-term survival between the transhiatal and transthoracic approaches (Table 38-1).16–18 Most of these were limited by small study size and selection bias. A large meta-analysis review was performed by Rindani and coworkers19 in 1999. Forty-four trials published in the English literature between 1986 and 1996 consisting of 5483 patients undergoing either Ivor Lewis or transhiatal esophagectomy were reviewed. The overall incidence of pneumonia was 25%, and it was not appreciably different between the two techniques. The incidence of bleeding and cardiac complications was also not different. The most significant differences were seen in the anastomotic leak rate (16% transhiatal versus 10% Ivor Lewis), stricture (28% transhiatal versus 16% Ivor Lewis), and recurrent nerve injury (11% transhiatal versus 5% Ivor Lewis). Perioperative mortality was higher in the Ivor Lewis population (9.5%) than in the transhiatal population (6.3%). Overall long-term (5-year) survival was similar in the two groups, 25%. Hulscher and colleagues20 also performed a meta-analysis of trials between 1990 and 1999 published in English language journals. Fifty publications were identified, some randomized, some comparing transthoracic with transhiatal approaches, and some evaluating only one technique. Overall, cardiac complications (20% versus 7%), anastomotic leakage (14% versus 7%), and vocal cord paralysis (10% versus 4%) were higher in the transhiatal versus the transthoracic groups. Pulmonary complications (19% versus 13%), in-hospital mortality (9% versus 6%), and operative time (5.6 versus 4.0 hours) were higher in the transthoracic versus the transhiatal group. Overall 5-year survival was similar among all the studies and patients evaluated (23% for transthoracic resections and 21.7% for transhiatal resections). These reviews are of historical and factual interest only, and little can be concluded about the relative merits of each technique in matched populations.
Table 38–1 Comparison of Transthoracic and Transhiatal Approaches to Esophagectomy: Perioperative Complications
| Complication | Transthoracic | Transhiatal |
|---|---|---|
| Blood loss (mL) | 1001 | 728 |
| Operative time (hours) | 5.6 | 4.0 |
| Cardiac complications (%) | 6.6 | 19.5 |
| Pulmonary complications (%) | 18.7 | 12.7 |
| Anastomotic leak (%) | 7.2 | 13.6 |
| Vocal cord paralysis (%) | 3.5 | 9.5 |
| Chyle leak (%) | 2.4 | 1.4 |
| In-hospital mortality (%) | 9.2 | 5.7 |
Modified from Hulscher J, Tijssen J, Lanschot J. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Cumulative review of all English literature publications (searched via MEDLINE) between 1990 and 1999 comparing transhiatal with transthoracic esophageal resection.
Orringer and colleagues21 reviewed their 30-year experience with transhiatal esophagectomies in 2007 patients. The patients were divided into two groups: group I, those operated on between 1976 and 1998; and group II, those operated on from 1998 to 2006. Postoperative mediastinal bleeding requiring reoperation occurred in less than 1%. The incidence of recurrent laryngeal nerve injury was significantly lower in group II (2%) compared with group I (7%; P < .0001). They believed that this decrease in injury was secondary to an increase in operative volume. Chylothorax was seen in 1%. The incidence of respiratory complications that required a hospital stay beyond 10 days was 2%. They also reported that the routine use of side-to-side stapled cervical esophagogastric anastomosis after 1997 has led to a notable decrease in leak rate and need for postoperative dilation. The overall hospital mortality was 3%. The hospital mortality fell with increased volume (group I, 4%; group II, 1%). It is clear that the high volume at this center has produced impressive results with regard to transhiatal esophagectomy.
Wolff and associates22 retrospectively reviewed all esophagectomies at the Mayo Clinic between 1994 and 2004. Of the 517 esophagectomies performed, the type of surgery was as follows: transhiatal (68), Ivor Lewis (392), and extended Ivor Lewis (57). The mean lymph nodes retrieved for the Ivor Lewis and extended Ivor Lewis were 18.7 and 17.4, respectively. This was not statistically different. The transhiatal patients had a mean of 8.99 lymph nodes in the specimen. They found that the combined Ivor Lewis group and the transhiatal group were significantly different, showing on average 9.5 lymph nodes in the surgical specimen (P < .001). The importance of the number of lymph nodes harvested at the time of surgery is discussed in a later section.
Orringer and associates23 reviewed outcomes after transhiatal and transthoracic surgery using the Surveillance, Epidemiology, and End Results (SEER) cancer registry. They identified patients between 1992 and 2002 undergoing esophagectomy. Of the 868 patients studied, 643 had a transthoracic approach and 225 underwent a transhiatal esophagectomy. They concluded that patients undergoing a transhiatal esophagectomy had a lower operative mortality rate than did those undergoing transthoracic operations (6.7% versus 13.1%; P = .009). The need for anastomotic dilation was higher in the transhiatal group (43.1%) compared with the transthoracic patients (34.5%; P = .02). The 5-year survival was significantly higher in the transhiatal group; however, this benefit over the transthoracic group was diminished after adjustments were made for patient and hospital or provider characteristics, such as volume performed at the center and involvement of trainees.
Three randomized, prospective trials comparing transhiatal resection with transthoracic resection demonstrated that either technique may be performed safely in skilled hands, and no clear differences have been shown between the two techniques. This is in large part due to an inadequate number of patients enrolled in the trials. Extrapolating from their retrospective meta-analysis, Rindani and colleagues19 estimated that 2360 patients would have to be randomized for a significant difference to be shown in perioperative mortality, and 6400 patients would be needed for a difference to be shown in long-term survival. Clearly, this is beyond the capacity of any prospective esophagectomy trial.
The first randomized, prospective trial was published in 1993 by Goldminc and coworkers.24 They randomized 67 patients younger than 70 years with squamous cell cancer of the esophagus to Ivor Lewis esophagectomy or transhiatal esophagectomy. Operative time was longer (6 versus 4 hours) in the transthoracic group. There was no difference in incidence of pneumonia (20%), anastomotic leak, recurrent nerve injury, bleeding, perioperative mortality, or length of hospitalization. At a mean follow-up of 3 years, survival was not statistically different between the two groups. For those patients with nodal disease, however, none of the transhiatal patients was alive at 18 months, whereas 30% of the transthoracic patients were alive at 18 months.
Wong and colleagues reported a prospective, randomized series of 39 patients with lower third esophageal cancers treated with either an Ivor Lewis resection or a transhiatal resection.25 Patients undergoing neoadjuvant therapy or those with an FEV1 below 70% were excluded from the study. There were no perioperative (30-day) deaths in either group, although the in-hospital mortality was 15% for the transhiatal group and 0% for the transthoracic group (not significantly different). Intraoperative hypotension occurred in 60% of transhiatal patients but in only 5% of transthoracic patients. There was no difference in blood loss, pneumonia, or recurrent nerve injury. Operating time was longer for the transthoracic group. The mean proximal margin was 3 cm longer in the transhiatal group. No significant difference was seen in tumor recurrence or survival. This group recently published their 5-year results from this study.26 After a complete 5-year follow-up, they concluded that overall survival was not significantly different between the two groups. They did report a trend toward improved survival in the extended transthoracic group over the transhiatal group in the patients with a type I esophageal cancer.
More recently, a randomized study was completed in the Netherlands comparing transhiatal resection with extended transthoracic en bloc resection for distal adenocarcinomas of the esophagus or cardia27; 106 patients had a transhiatal resection, and 114 patients had a transthoracic resection. The median follow-up was 4.7 years. In-hospital mortality was 2% to 4% in each group. Respiratory complications including atelectasis and pneumonia were higher in the transthoracic group (57% versus 27%), as was the incidence of chyle leak (10% versus 2%). The high incidence of respiratory complications in the transthoracic group (57%) should be questioned, as it is much higher than that quoted in many prior transthoracic resection series.19 Although statistical significance was not reached, there was a trend toward improved survival at 5 years in the transthoracic group (39% versus 29%).
Minimally Invasive Esophagectomy
Minimally Invasive Tri-incisional Esophagectomy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree