Esophageal Motility Disorders
Andrea S. Wolf
Cameron D. Wright
Esophageal motility disorders are uncommon and usually present with dysphagia or its sequelae (regurgitation and aspiration) or atypical noncardiac chest pain. These disorders are diagnosed by esophageal manometric studies that assess lower esophageal sphincter (LES) pressure and relaxation and the nature of esophageal contraction waves, including amplitude, duration, repetitive nature, the presence of nontransmitted or partially transmitted waves, and the presence of peristalsis in the body of the esophagus. Esophageal motility disorders are classified as primary when not related to a systemic disease and secondary if they are associated with a systemic disease. In a series published by Patti et al.,49 11.4% of the 3,471 patients referred to their swallowing center for evaluation were diagnosed with a primary esophageal motility disorder. Table 149-1 lists the primary motility disorders with their manometric profiles. Reviews emphasizing the manometric diagnosis and medical management of the primary esophageal motility disorders have been published by Spechler and Castell66 and Richter.60
Achalasia
Achalasia, a term of Greek origin, means failure to relax. Achalasia was first described and effectively treated (by dilation with whalebone) by Thomas Willis in 1679. The classic findings on manometry are failure of the LES to relax in response to a swallow and absent peristalsis in the smooth muscle of the distal esophagus. The etiology of achalasia is unknown. Possible causes include hereditary, degenerative, autoimmune, and infectious etiologies. Achalasia is rare, with an incidence of only about 0.5 per 100,000 people. Although described in both the very young and elderly people, it occurs most commonly between the ages of 20 and 50 years. The gender distribution is equal.
History
Payne50 reviewed the history of the surgical treatment of achalasia with an emphasis on Heller’s contribution. Surgical treatment before the acceptance of Heller’s technique was by a variety of anastomotic cardioplasties that relieved obstruction but led to severe esophagitis, with its attendant complications. In 1914, Heller reported a successful result in a patient treated with a transabdominal double (anterior and posterior) esophagomyotomy. His operation did not become standard until the devastating late effects of severe reflux disease were reported by Barrett and Franklin in 1949. Heller’s operation was modified to a single anterior esophagomyotomy by Groeneveldt in 1918 and further popularized by Zaaijer in 1923.
Pathophysiology
Pathologic studies have shown abnormalities in the esophageal myenteric (Auerbach’s) plexus, which include inflammation, loss of ganglion cells, and fibrosis. Degenerative changes of the vagus nerve and changes of the dorsal motor nucleus of the vagus have also been described. Central vagal dysfunction and destruction of the peripheral myenteric plexus are the two hypotheses that could explain achalasia; it is unclear which is correct. The end result of the destruction of the myenteric plexus is a selective loss of postganglionic inhibitory neurons containing nitric oxide and vasoactive intestinal polypeptide, which leads to impaired LES relaxation. Loss of inhibitory input mediated by nitric oxide leads to loss of peristalsis. Postganglionic cholinergic neurons are spared, and cholinergic stimulation continues unopposed, leading to high LES pressures.
Diagnosis
Clinical Features
Patients with achalasia usually have dysphagia to solids and liquids (76%), as reported by Blam and associates.6 Regurgitation is frequent (79%) and may contain food or saliva. Regurgitation is especially frequent at night during recumbency and may cause coughing or choking spells. Most patients (79%) learn to eat slowly and may have adaptive mechanisms such as repetitive swallows with the neck extended and using liquids to lubricate solid food. Chest pain occurs in some patients, especially those with early disease. Weight loss is common but not always present. Obesity does not eliminate the possibility of achalasia. A history of food bolus impaction may be present. Heartburn is often present as a symptom (24%), although because the LES does not relax normally, the cause is not classic gastroesophageal reflux. It is thought to stem from the lactic acid from the fermented retained food and impaired clearance of refluxed acid. Pulmonary symptoms may predominate, with occasional patients diagnosed after hospitalization for aspiration pneumonia. Most patients live with their symptoms for years, and misdiagnosis of early cases is frequent.
Table 149-1 Classification of Primary Esophageal Motility Disorders and Their Manometric Features | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Radiographic Evaluation
Chest radiographs may show subtle findings such as an esophageal air–fluid level, absent gastric air bubble, abnormal mediastinal contour, or even evidence of aspiration pneumonia. Barium swallow may show the characteristic findings of a dilated esophagus and a smooth, tapered distal narrowing at the gastroesophageal junction (“bird’s beak” appearance). See Chapter 133 for a more detailed discussion of contrast study and fluoroscopic findings in achalasia.
Endoscopy
An upper gastrointestinal endoscopy is necessary in the diagnostic evaluation to exclude mucosal diseases that could mimic achalasia, especially cancer of the esophagogastric junction (pseudoachalasia). In achalasia, the body of the esophagus is usually dilated and often has retained food and liquid. Retention esophagitis may be present, with a cobblestone appearance to the mucosa. The LES is usually tonically closed, but it is possible to pass the endoscope with gentle pressure through the esophagogastric junction. Cameron and colleagues9 have reported on the accuracy of video endoscopy to diagnose achalasia by noting the response of the visualized esophagus to a swallow. In almost all patients, there was an absence of lumen-occluding contractions with failure of the LES to open. A correct diagnosis was made in 96% of the patients. The LES should feel “soft” as it is traversed. If passage is difficult and there is the suggestion of unusual firmness, a cancer should be suspected and an endoscopic ultrasound performed. If pseudoachalasia is suspected, a computed tomography (CT) scan should also be performed to look for a mass at the esophagogastric junction.
Manometry
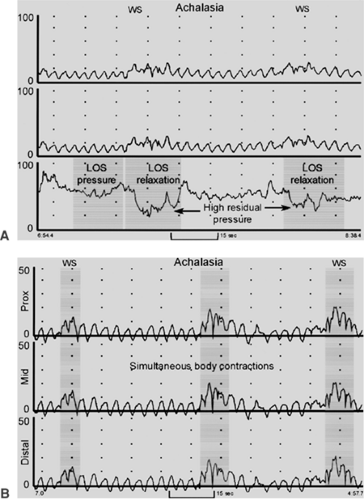
Manometry is the key diagnostic test because endoscopic and radiographic exams are never diagnostic of achalasia. The manometric abnormalities in achalasia are always confined to the distal esophagus because only the smooth muscle is affected. The two manometric features required to make a diagnosis of achalasia are (a) incomplete relaxation of the LES and (b) aperistalsis in the body of the esophagus characterized by either simultaneous low-amplitude esophageal contractions (<40 mm Hg) or no esophageal contractions (Fig. 149-1). The LES pressure is usually high (never low) but can be normal (10 to 45 mm Hg). Absent or incomplete LES relaxation is seen in about 80% of patients. In the remaining 20%, the LES relaxes to the gastric baseline, but this is of abnormally short duration (<6 seconds), leading to functional obstruction. The term vigorous achalasia is used if high-amplitude (>50 mm Hg) simultaneous contraction waves are present in the body of the esophagus. The resting pressure in the body of the esophagus is often noted to be elevated (“pressurized”) above that of the gastric baseline.
Treatment
There is no curative treatment for achalasia because it is impossible to restore peristaltic function to the denervated esophagus. Current treatment is directed at reducing the pressure gradient across the LES, thus reducing outflow obstruction and allowing gravity to empty the esophagus. The two most effective treatments are mechanical: balloon dilation and surgical myotomy. Pharmacologic treatment is less effective and includes injection of botulinum toxin into the LES and orally administered drugs that reduce LES pressure. Esophageal resection is rarely needed for failure of primary therapy.
Medical Therapy
Several types of drugs are effective at reducing LES pressure; these include beta agonists, anticholinergics, nitrates, and calcium channel blockers. The effects of sildenafil (Viagra), a phosphodiesterase inhibitor, on the esophagus were reported by Eherer et al.21 All of these medications have been used in achalasia, but all are of limited use because of either poor efficacy or adverse side effects.
Botulinum Toxin Injection
Pasricha and associates46 reported favorable results of the first trial of botulinum toxin injection in patients with achalasia in 1994. Botulinum toxin inhibits acetylcholine release from nerve endings, thereby reducing the cholinergic drive to the LES, with resultant sphincter relaxation. Over time, neurotransmission and muscle activity recover after new nerve endings are sprouted and new synapses to adjacent muscle fibers are formed. Botulinum toxin is administered on an outpatient basis via an endoscope through a sclerotherapy needle. Usually, 25 U is delivered into each of the four quadrants of the cardia in 1-mL aliquots. The initial success rate is about 70%, with symptom relief lasting up to several years. The only common side effects are transient chest pain after the procedure and reflux symptoms. Neubrand and coworkers42 reported on medium-term results and prognostic factors with botulinum toxin injection. The LES pressure was significantly reduced from 62 to 43 mm Hg after treatment. Thirty-six patients had symptomatic relief a mean of 2.5 years after treatment, whereas 64% had a good initial result. Retreatment was of no benefit if results were assessed at 6 months. Patients with high LES pressures (mean 73 mm Hg) and younger patients (mean 46 years) had poor results. In a randomized controlled trial comparing 40 patients assigned to a set of two botulinum toxin injections one month apart with another 40 patients assigned to laparoscopic Heller myotomy, Zaninotto et al.74 found that initial response to treatment was excellent and similar in both groups. Symptoms recurred in the botulinum group, however, for 40% of patients by 1 year and 66% by 2 years.
Botulinum toxin injection is best suited for high-risk elderly patients with low LES pressures. Retreatment is of some value if there was an initial good response to injection but is of little benefit if the initial response was poor. Increasing the dose of botulinum toxin has been of no benefit in improving the response.
Many believe that botulinum toxin injection may complicate the results of later myotomy, although studies have not shown a clear relationship. Raftopoulos and colleagues53 found that myotomy patients who had undergone prior botulinum toxin injection did not have significant improvement in symptoms of heartburn, regurgitation, chest pain, or sense of well-being, while those without prior nonoperative treatment did improve in all of these areas. Nevertheless, the group that had undergone prior botulinum toxin injection did have significantly improved dysphagia scores, and the same study showed no significant effect of prior nonoperative treatment on surgical outcome. Patti et al.48 attributed two of their myotomy failures (and intraoperative perforations) to transmural fibrosis from prior botulinum toxin injection. In contrast, Deb and coworkers15 found that prior botulinum toxin injection was not associated with intraoperative perforation or poor functional outcome. Similarly, in comparing myotomy patients who had undergone prior endoscopic treatment for achalasia (including 34 patients who had undergone prior botulinum injection) with those who had not, Perrone et al.51 found no significant difference in the rate of intraoperative perforation, myotomy failure, postoperative solid dysphagia score, or overall patient satisfaction. Whether botulinum therapy might interfere with later myotomy remains a controversial issue.
Pneumatic Dilation
Forceful dilation of the LES is a noninvasive, time-tested treatment option in achalasia. Originally a large, relatively compliant bag dilator (Mosher bag) was swallowed and positioned across the cardia and inflated with contrast material under fluoroscopic control until the “waist” of the LES impression disappeared. The aim of pneumatic dilation is rupture of the muscle fibers of the LES with maintenance of an intact esophageal mucosa. The reason this seemingly contradictory result can occur is the different compliances of the esophageal sphincter (less compliant) and the mucosa (more compliant). Good results were obtained initially in about 70% of patients, with a perforation rate of 2% to 5%. More recently, balloon dilation has been performed with polyethylene noncompliant-sized balloons that can be passed through the working channel of an endoscope or over a guidewire. The most commonly used balloons are the Rigeflex balloons (Microinvasive, Watertown, MA) in 3-, 3.5-, and 4-cm sizes. The procedure is usually done on an outpatient basis with sedation. Most patients have rather significant chest pain during the dilation, which rapidly improves. If the pain continues, the patient should be evaluated for perforation. Most gastroenterologists do the dilation under endoscopic but not radiographic control. Results are then judged by relief of dysphagia in follow-up. If there is inadequate relief, the next size of balloon dilator is used. A more precise technique is to use radiographic control with inflation of the balloon with dilute contrast; dilation is continued until the waist of the LES disappears. Kadakia and Wong29 reviewed the results of modern balloon dilation. The symptomatic response rate was 74% to the 3-cm balloon, 33% to the 3.5-cm balloon, and 5% to the 4-cm balloon. The perforation rate increased as balloon size increased: 1% with the 3-cm and 15% with the 4-cm balloon. In the short term, relapse occurs at about 6% per year. Troublesome heartburn occurred in only 5% of patients. Sabharwal and colleagues63 reported results in 76 patients using radiographic control of balloon dilation. There were no perforations, and 89% of patients reported satisfactory improvement of their swallowing. Fifty-two patients required a
single dilation, 22 patients between two and four dilations, and 2 patients needed five dilations. There is very little information on the long-term results of dilation. West and coworkers71 recently reported long-term results in patients after dilation. At 5 years, the success rate was only 50%, and at 15 years, the success rate dropped to only 40%. Of the 32 patients who died during the study, 6 (19%) died of esophageal cancer. Sabharwal and associates63 reported safe, effective balloon dilation after failed surgical myotomy. Alternatively, Ferguson and colleagues23 and Dolan and coworkers19 reported good results with surgical myotomy after failed balloon dilation. Balloon dilation is usually offered as first-line therapy by gastroenterologists because of its minimally invasive nature, good initial results, and low cost compared with myotomy.
single dilation, 22 patients between two and four dilations, and 2 patients needed five dilations. There is very little information on the long-term results of dilation. West and coworkers71 recently reported long-term results in patients after dilation. At 5 years, the success rate was only 50%, and at 15 years, the success rate dropped to only 40%. Of the 32 patients who died during the study, 6 (19%) died of esophageal cancer. Sabharwal and associates63 reported safe, effective balloon dilation after failed surgical myotomy. Alternatively, Ferguson and colleagues23 and Dolan and coworkers19 reported good results with surgical myotomy after failed balloon dilation. Balloon dilation is usually offered as first-line therapy by gastroenterologists because of its minimally invasive nature, good initial results, and low cost compared with myotomy.
Esophagomyotomy
Esophageal myotomy is the definitive treatment for achalasia. The goals of the procedure are to reduce the LES pressure enough to allow gravity drainage of the esophagus and paradoxically maintain (or augment) control of gastroesophageal reflux. Heller’s original technique was a double myotomy through a laparotomy. This was abandoned because of late severe reflux complications. A single myotomy is now accepted by all as allowing enough reduction in LES pressure to relieve outflow obstruction. Many South American and most European surgeons continued with the laparotomy approach because, in those areas historically, visceral surgeons usually operated on the esophagus. American thoracic surgeons who have historically operated on the esophagus naturally approached a myotomy through the chest. With the advent of minimally invasive surgery in the early 1990s, esophageal myotomy was performed with video-assisted thoracic surgery (VATS) techniques. This approach had several disadvantages: the myotomy must be approached perpendicularly, single-lung ventilation is required intraoperatively, and chest drainage is required postoperatively.28 Ramacciato and associates54 compared the VATS and the laparoscopic approaches to myotomy within their own unit. Laparoscopic myotomy was found to be superior in several areas: shorter length of surgery, shorter length of stay, better dysphagia relief, less postoperative heartburn, and less incisional discomfort. Table 149-2 summarizes the recent studies on laparoscopic Heller myotomy.
Conversion to an open procedure is very rare. Intraoperative mucosal injury occurs in about 5% of patients and can almost always be handled with laparoscopic suturing and buttressing with an anterior Dor wrap. There are various techniques to perform the myotomy; it is not clear which is best. Myotomy can be performed with scissors, hook and cautery division, or by tearing muscle fibers between two forceps. It is very important not to injure the mucosa with the cautery, which could present as a delayed perforation. We prefer the scissors. Almost all bleeding from muscular vessels will stop with time and pressure during the myotomy, which helps to avoid the need for cauterization. Many surgeons obtain a control barium swallow the following morning to document the relief of LES obstruction and the absence of a leak, although authors of several large series report abandoning the routine use of postoperative barium swallow, citing extremely low yield and no effect on postoperative management. Liquids are allowed the first day and rapidly advanced to a soft diet. The average length of stay is 1 to 2 days. Most series report good results in 90% to 95% and heartburn in 5% to 25%. Well into the second decade of this technique, there are now long-term data demonstrating continued successful outcome. Wright and colleagues73 followed, for a median of 63 months, 30 of their patients who underwent laparopscopic Heller myotomy and found no significant worsening of patient dysphagia during that period. After a mean follow-up of 95 months, Costantini and coworkers12 found that although symptom scores with regard to dysphagia, regurgitation, and chest pain worsened over time, they were all still significantly improved from preoperative values. Furthermore, they found that more than half of the episodes of symptom recurrence appeared within the first year postoperatively. Although most surgeons consider laparoscopic Heller myotomy the surgical treatment of choice for achalasia, controversy still exists regarding the length of the gastric portion of the myotomy and which if any antireflux repair should be performed.
Whether to include an antireflux procedure is a highly debated issue. In a meta-analysis of 21 studies from 1995 to 2000, Lyass et al.37 compared 532 patients who underwent laparoscopic Heller with fundoplication to 69 patients without fundoplication. There was no significant difference in
the severity of gastroesophageal reflux symptoms or the rate of reflux detected by pH monitor between the two groups, although there was a trend toward a lower rate of reflux on pH probe studies in the group with fundoplication. Recurrent postoperative dysphagia occurred in 1.5% of the nonfundoplication patients, with one patient requiring reoperation, and in 3.2% of the fundoplication patients, with three requiring takedown of the fundoplication, two requiring myotomy revision, and one ultimately requiring esophagectomy. In a recent study with short-term follow-up by Finley and colleagues,24 24 patients who underwent laparoscopic Heller without fundoplication had greater improvement in esophageal clearance time (by nuclear study) than did 71 patients whose procedure included a Dor fundoplication. The authors noted, however, that there was significantly worse esophageal clearance in the no-wrap group compared with the Dor group preoperatively, perhaps allowing more “room for improvement.” Nevertheless, there were no significant differences in the symptom scores for dysphagia, regurgitation, or heartburn between the two groups postoperatively. Bloomston and Rosemurgy7 reported their experience using fundoplication only for specific indications: to buttress an esophageal repair or to manage a patulous hiatus or hiatal hernia. They found no significant difference in postoperative dysphagia scores between patients who had fundoplication and those who did not. Rice et al.55 found that patients with fundoplication (N = 88) had higher resting and residual LES pressures postoperatively than those without fundoplication (N = 61). The investigators did not report patients’ symptoms to corroborate the clinical impact of these data. The no-wrap group had more time of pathologic reflux (percentage of time with pH <4 on 24-hour pH probe) while supine postoperatively than the wrap group, although there was no significant difference in percentage of time with pathologic reflux while upright.
the severity of gastroesophageal reflux symptoms or the rate of reflux detected by pH monitor between the two groups, although there was a trend toward a lower rate of reflux on pH probe studies in the group with fundoplication. Recurrent postoperative dysphagia occurred in 1.5% of the nonfundoplication patients, with one patient requiring reoperation, and in 3.2% of the fundoplication patients, with three requiring takedown of the fundoplication, two requiring myotomy revision, and one ultimately requiring esophagectomy. In a recent study with short-term follow-up by Finley and colleagues,24 24 patients who underwent laparoscopic Heller without fundoplication had greater improvement in esophageal clearance time (by nuclear study) than did 71 patients whose procedure included a Dor fundoplication. The authors noted, however, that there was significantly worse esophageal clearance in the no-wrap group compared with the Dor group preoperatively, perhaps allowing more “room for improvement.” Nevertheless, there were no significant differences in the symptom scores for dysphagia, regurgitation, or heartburn between the two groups postoperatively. Bloomston and Rosemurgy7 reported their experience using fundoplication only for specific indications: to buttress an esophageal repair or to manage a patulous hiatus or hiatal hernia. They found no significant difference in postoperative dysphagia scores between patients who had fundoplication and those who did not. Rice et al.55 found that patients with fundoplication (N = 88) had higher resting and residual LES pressures postoperatively than those without fundoplication (N = 61). The investigators did not report patients’ symptoms to corroborate the clinical impact of these data. The no-wrap group had more time of pathologic reflux (percentage of time with pH <4 on 24-hour pH probe) while supine postoperatively than the wrap group, although there was no significant difference in percentage of time with pathologic reflux while upright.
In a landmark prospective study, Richards and coworkers56 randomized patients to laparoscopic myotomy with (N = 22) and without (N = 21) Dor fundoplication and followed their symptoms as well as manometric and 24-hour pH probe results postoperatively. There was no significant difference in
postoperative dysphagia between the groups. The nonfundoplication patients had higher median acid exposure time, higher number of episodes of acid exposure, and higher percentage time of acid exposure both supine and upright. Although these are impressive data, their clinical impact—for example, on the incidence of peptic stricture or Barrett’s transition—is unknown. Moreover, the incidence of pathologic gastro- esophageal reflux disease (GERD) (as defined by >4.2% of a 24-hour period with pH <4) in nonfundoplication patients in this study (47.6%) was markedly higher than that in other series, including one by the same group.57 The authors attribute this difference to the more recent study involving a younger patient population with lower LES pressures; they had found these two risk factors to be associated with the development of pathologic GERD after Heller myotomy. In an interesting report, the same group performed a cost–utility analysis based on 1-year follow-up data from their randomized trial.69 They used the Markov simulation model to project cost and adjust quality of life for postoperative GERD and dysphagia. Although the operative cost of the Dor group was higher owing to longer operating room time, projected costs at 10 years showed Dor to be cost-effective when the cost of proton pump inhibitor therapy was taken into account ($6,800 versus $9,500 per patient over 10 years).
postoperative dysphagia between the groups. The nonfundoplication patients had higher median acid exposure time, higher number of episodes of acid exposure, and higher percentage time of acid exposure both supine and upright. Although these are impressive data, their clinical impact—for example, on the incidence of peptic stricture or Barrett’s transition—is unknown. Moreover, the incidence of pathologic gastro- esophageal reflux disease (GERD) (as defined by >4.2% of a 24-hour period with pH <4) in nonfundoplication patients in this study (47.6%) was markedly higher than that in other series, including one by the same group.57 The authors attribute this difference to the more recent study involving a younger patient population with lower LES pressures; they had found these two risk factors to be associated with the development of pathologic GERD after Heller myotomy. In an interesting report, the same group performed a cost–utility analysis based on 1-year follow-up data from their randomized trial.69 They used the Markov simulation model to project cost and adjust quality of life for postoperative GERD and dysphagia. Although the operative cost of the Dor group was higher owing to longer operating room time, projected costs at 10 years showed Dor to be cost-effective when the cost of proton pump inhibitor therapy was taken into account ($6,800 versus $9,500 per patient over 10 years).
Table 149-2 Recent Studies on Laparoscopic Heller Myotomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree