Esophageal Endoscopic Procedures in Thoracic Surgery
Susan Ramdhaney
Scott Tenner
Background
Over 14 million adults suffer from gastroesophageal reflux disease (GERD) annually. The pathogenesis of GERD involves the inappropriate relaxation of the lower esophageal sphincter. This allows reflux of gastric contents, including acid, into the esophagus. Other than heartburn, this refluxate can lead to strictures, ulcers, and Barrett’s esophagus, a precursor to esophageal adenocarcinoma. Whereas heartburn will affect most persons at some time during their lives, 7% to 10% of the population will suffer from the symptoms for years, requiring medical therapy. Although most patients will respond to medical therapy with proton pump inhibitors (PPIs), some will have difficulty affording the cost, and the therapy will not be effective in a significant proportion of patients. For this reason, minimally invasive endoscopic interventions have been devised to help patients with diseases of the esophagus, especially GERD.
Endoluminal Therapies for the Treatment of Gerd
A myriad of endoscopic treatments have been proposed for the treatment of gastrointestinal reflux disease.21 In the year 2000, the U.S. Food and Drug Administration (FDA) gave approval to the first two devices for the endoscopic treatment of GERD. These devices, known as EndoCinch and Enteryx, have herald the invention of numerous others designed to reduce reflux symptoms and the need for PPI therapy. Their intent is to alter the gastroesophageal junction (GEJ) and hence decrease the number of reflux events and/or the volume of the refluxate. One proposed mechanism is the induction of fibrosis and surrounding tissue changes at the GEJ, resulting in anatomic/structural changes such as tightening, lengthening, thickening, and narrowing and thus a reduction in the number of reflux events. In addition, a decrease in the compliance and distensibility of the GEJ results in a decrease in the frequency of transient lower esophageal sphincter relaxation (tLESR), thus also reducing reflux. It has also been proposed that recently developed methods of using radiofrequency energy may serve to ablate neural elements in the area of the GEJ and thus trigger decreased tLESR2 (Table 153-1).
Endoluminal Gastroplication
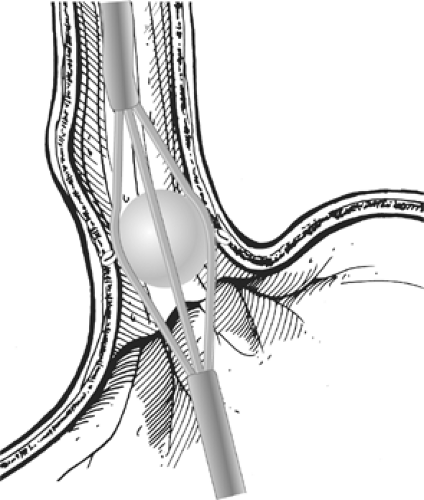
The Bard EndoCinch Suturing System was one of the first endoluminal procedures approved by the FDA. Approved in May 2000, this suturing apparatus is attached to the tip of an endoscope in order to place multiple sutures just below the gastro- esophageal junction. By pulling together the folds of the upper stomach, creating fundoplications or “pleats” within the lower esophageal sphincter (LES), this procedure is intended to decrease the reflux of gastric contents into the distal esophagus. It uses two endoscopes and is a rather complex process. First an esophageal overtube is placed over an esophageal dilator, the dilator is removed, and the overtube is left in place for the multiple endoscopic passes needed to create each plication. With a second endoscope, the gastroenterologist advances the EndoCinch suturing device to approximately 1 to 2 cm below the squamocolumnar junction. By application of 10 to 20 second wall suction, a fold of tissue is suctioned into the EndoCinch capsule chamber, a needle and suture are advanced through the tissue, and a stitch is placed through the suctioned tissue. The scope is withdrawn to pull the suture through the tissue. The suture is then reloaded into the needle and the endoscope is reinserted to the stitch location for the placement of the second
stitch 1 cm adjacent to the first stitch. After repeated stitches, the endoscope with the suture anchor system creates a plication, which serves as a tightened LES (Figs. 153-1 and 153-2).
stitch 1 cm adjacent to the first stitch. After repeated stitches, the endoscope with the suture anchor system creates a plication, which serves as a tightened LES (Figs. 153-1 and 153-2).
Table 153-1 Endoluminal Therapies for GERD | |
|---|---|
|
Multiple studies have been performed to evaluate this technique. Mahmood et al.5 showed that with the EndoCinch, the use of PPIs was reduced by 64%, with symptomatic improvement in reflux patients. However, questions about the stitch durability and the laborious nature of the procedure have resulted in the device losing its popularity. In addition, a small population has been reported to develop dysphagia as a complication, while some studies have reported few cases of “significant” bleeding.
Stretta Device
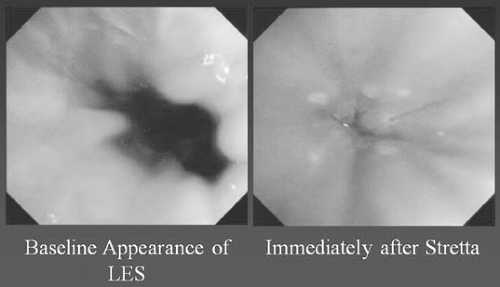
The Stretta6 procedure is another approved technique for the treatment of GERD.21 This device delivers controlled thermal energy to the muscular layers of the esophagus and the resulting injury heals, leaving scar tissue in the wall of the esophagus. The resultant tissue constriction from the scar results in improved muscle wall thickness. This increases the barrier function at the LES by decreasing the frequency of transient lower esophageal sphincter relaxations (tLESRs), and hence decreasing GERD (Fig. 153-3).
In addition, the Stretta procedure has been demonstrated to increase lower esophageal sphincter pressure, decrease esophageal acid exposure time, and decrease the need for acid-blocking drugs, thereby controlling GERD symptoms and esophagitis. Significant improvements in physical and mental quality-of-life scores of GERD patients have also been noted after the Stretta procedure. In a randomized blinded, sham crossover trial, the Stretta device was shown to be clinically more effective in preventing GERD symptoms than the sham arm. When crossed over to the Stretta arm, patients who had previously had no clinical benefit experienced a significant decrease in GERD symptoms. However, it is unclear whether this was related to decreased acid reflux, as the esophageal pH was not significantly different between both groups.
Post-Stretta adverse events were limited to transient fever, dysphagia, chest pain, superficial mucosal injury, and anesthesia reactions. There were no new cases of swallowing problems, achalasia, or decreased esophageal peristalsis (motility) reported after this procedure. However, it is not recommended for patients with severe GERD, large hiatal hernias, or a poorly functioning esophagus with dysphagia or stricture. Ideal candidates for the Stretta procedure include patients with a desire not to continue GERD medications, patients who experience drug intolerance, or those with inadequate symptom response. In these patients, endoscopic radiofrequency electrosurgery can be offered as an alternative to long-term drug therapy or fundoplication. Some studies have even shown that patients treated with the Stretta device are highly satisfied and have improved GERD symptoms and quality of life when compared with patients undergoing laparoscopic fundoplication (Fig. 153-4).8
Enteryx Copolymer Injection Procedure
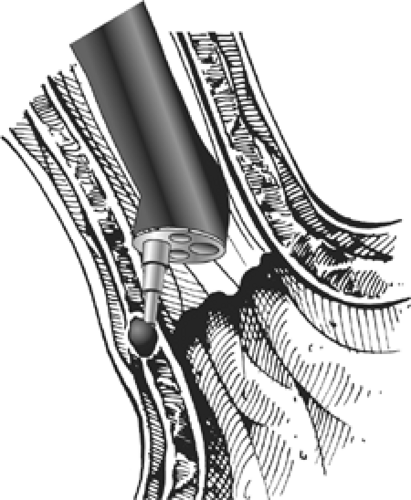
Enteryx is an injectable solution of 8% ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide and mixed with tantalum to render it radiopaque. On contact with polar physiologic fluid, the dimethyl sulfoxide diffuses away and the hydrophobic copolymer precipitates and forms a spongy solid mass. This substance, originally developed as an embolic agent, was shown to be safe in terms of both local and systemic effects in the preclinical phase of testing, during which the feasibility of this approach for the treatment of GERD and the durability of the implant were also demonstrated.24 Histologically, after injection of Enteryx into tissue, acute inflammation leads to fibrosis, followed by a well-developed foreign-body reaction (Fig. 153-5).8
The main advantages of Enteryx injection over the other FDA-approved GERD endotherapies is that it is a technically simple procedure and makes use of familiar endoscopic skills, adding minimal extra time to the average upper endoscopy.22 However, because of the frequency needed to repeat implantation, the degree of radiation exposure to the patient, questions about the optimal site of delivery, intramural depth, injection volume, implant number, implant consistency and radial distribution, and the ability to remove the implant if desired, this treatment has been less preferred.
The efficacy of Enteryx implantation has been evaluated in a prospective, multicenter, single-arm study.24 After 6 months of treatment, the use of PPI therapy was eliminated in 74% and reduced by more than 50% in 10% of the patients. However, Enteryx has fallen out of favor because of issues regarding its safety7 and the development of complications such as
pneumomediastinum28 and esophageal abscess.43 On September 23, 2005, despite its use in over 2,600 patients, Enteryx treatment was withdrawn from clinical practice by the manufacturer because the “efficacy of the application methods utilized in administering Enteryx are being investigated.”
pneumomediastinum28 and esophageal abscess.43 On September 23, 2005, despite its use in over 2,600 patients, Enteryx treatment was withdrawn from clinical practice by the manufacturer because the “efficacy of the application methods utilized in administering Enteryx are being investigated.”
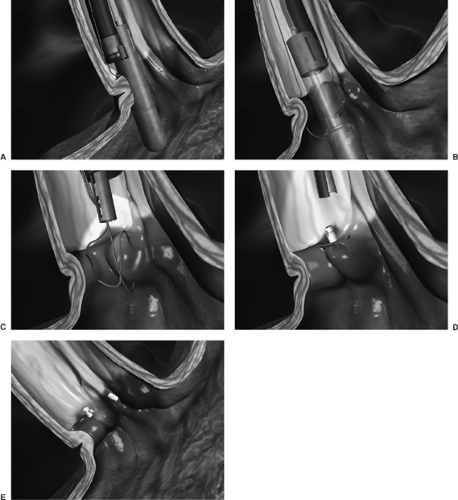 Figure 153-2. EndoCinch suturing device. Several plications are placed, creating a “flap” to prevent reflux of gastric contents. (Courtesy of Bard Endoscopic Technologies, Belleri, MA.) |
There is still much room for improvement in utilizing these periendoscopic devices for endoluminal therapy of GERD. To date, none have completely fulfilled the criteria of safety, efficacy, cost, durability, and reversibility. Studies continue to compare these therapies. For example, Domagh et al.,10 in comparing the efficacy of the EndoCinch 11 suturing system (C.B. Bard, Inc., Murray Hill, NJ) with that of the Enteryx copolymer injection technique (Boston Scientific Corp, Natick, MA) have concluded, after a 6-month follow-up study, that both methods have reduced GERD symptoms and the need for PPIs. However they and other investigators continue to show that none of these
techniques have improved LES tone enough to lead to the adoption of either technique as a preferred therapy.10
techniques have improved LES tone enough to lead to the adoption of either technique as a preferred therapy.10
Endoscopic Treatments for Barrett’s Esophagus
In a certain proportion of patients, acid reflux will lead to the metaplasia of the normal squamous epithelium of the esophagus. This metaplastic change has a salmon-colored appearance; such gastric-appearing mucosa in the esophagus is referred to as Barrett’s esophagus. This Barrett’s metaplasia, when accompanied by goblet cell formation and intestinal metaplasia, increases the risk of esophageal adenocarcinoma. The increased risk for developing adenocarcinoma has been estimated to be 0.5% per year. Prior to the development of adenocarcinoma, the cells undergo a process of dysplasia, from low grade to high grade, eventuating in carcinoma. Removal of dysplastic tissue is thought to decrease the risk of adenocarcinoma. Studies are ongoing to evaluate the efficacy of removing Barrett’s mucosa in an attempt to prevent future carcinoma.
Endoscopic mucosal resection (EMR) has become a promising treatment of dysplasia and early carcinoma in Barrett’s esophagus because it is associated with low morbidity and mortality.25 This endoscopic technique is usually performed for
focal lesions of dysplasia and/or carcinoma in Barrett’s esophagus or for complete removal of short-segment Barrett’s esophagus. The technique uses a small cap with a wire loop at the end of the endoscope. The lesion is then suctioned into the cap and the wire loop is closed while cautery is applied. The tissue can be examined under a microscope to determine if all of the cancer (or dysplasia) has been removed. Severe dysplasia and thin (≤2 mm) mucosal cancer of Barrett’s esophagus can be completely ablated (Fig. 153-6).
focal lesions of dysplasia and/or carcinoma in Barrett’s esophagus or for complete removal of short-segment Barrett’s esophagus. The technique uses a small cap with a wire loop at the end of the endoscope. The lesion is then suctioned into the cap and the wire loop is closed while cautery is applied. The tissue can be examined under a microscope to determine if all of the cancer (or dysplasia) has been removed. Severe dysplasia and thin (≤2 mm) mucosal cancer of Barrett’s esophagus can be completely ablated (Fig. 153-6).
The first report of localized EMR for Barrett’s esophagus in a cohort of 64 patients37 showed that for low-risk patients (lesion diameter <20 mm, well or moderately differentiated adenocarcinoma, or high-grade dysplasia limited to the mucosa), EMR could achieve complete remission in 97% of cases. For high-risk patients (lesion diameter >20 mm and limited to the mucosa; and/or poorly differentiated adenocarcinoma; and/or submucosal involvement) complete remission was achieved in 59%. Apart from a case of bleeding needing endoscopic therapy, no severe complications occurred. Over a mean follow-up period of 12 months, recurrent or metachronous carcinomas were found in 14% of these cases.11 The same group subsequently published data for a longer period of follow-up. Over a mean period of 34 months, higher recurrence rates of 23% to 30% were noted.36,38 Similar results have been published by other groups.5,32 Hence a major limitation of localized EMR is local recurrence. This is especially so for long-segment Barrett’s esophagus, even if histology shows complete removal of the malignant lesion. The high recurrence rate is due to the existence of multifocal premalignant and malignant areas in the Barrett’s esophagus overlooked by biopsy before EMR as well as metachronous development of new foci of dysplasia. Localized EMR has been combined with photodynamic therapy and argon plasma coagulation to address this issue of remnant Barrett’s esophagus, and studies show promising results.18,27,29 However, as in the case of ablative therapy alone, there remains serious concern over the lack of histologic assessment and the distinct possibility of incomplete ablation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree