CHAPTER 34 Esophageal Anatomy and Function
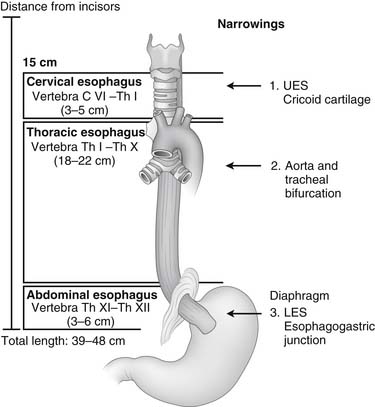
The esophagus is a muscular tube that starts as the continuation of the pharynx and ends as the cardia of the stomach. Knowledge of the anatomy of the esophagus and its relationship with other organs and structures is essential for the surgeon to evaluate the location of lesions seen by endoscopy, barium swallow study, or computed tomography; to interpret esophageal function studies; and to safely expose the esophagus during surgery (Fig. 34-1). Similarly, a knowledge of foregut embryology is key to the understanding of the pathogenesis of congenital malformations of the esophagus.
EMBRYOLOGY AND ANATOMY
The embryonic esophagus forms when paired longitudinal grooves appear on each side of the laryngotracheal diverticulum. These grooves subsequently grow medially and fuse to form the tracheoesophageal septum. This septum divides the foregut into the ventral laryngotracheal tube and the dorsal esophagus. Incomplete fusion of the two lateral grooves was formerly thought to be the major factor in the pathogenesis of congenital tracheoesophageal fistula, but the anomaly is now attributed to abnormal growth and differentiation of the lung buds. The esophagus is initially short, but it rapidly elongates; the relative final length is attained by the seventh week of gestation.1 This is followed by endodermal proliferation, which nearly obliterates the esophageal lumen. Subsequent recanalization occurs by the development of large vacuoles that coalesce. The adult position of the vagal nerves on the lower third of the esophagus results from the unequal growth of the greater and lesser curvature of the stomach, so the left vagus rotates anteriorly and the right vagus posteriorly.
The cricopharyngeal sphincter and the most proximal 1 to 2 cm of the cervical esophagus are primarily striated muscle. The striated muscle is derived from the caudal branchial arches and innervated by the vagus nerve and its recurrent laryngeal branches. The cricopharyngeal sphincter is made up primarily of the cricopharyngeus muscle, but it is also aided by the inferior pharyngeal constrictors and the circular muscles of the upper esophagus. Studies have indicated that the transition from predominantly striated to predominantly smooth muscle occurs in the proximal 4 to 5 cm of the esophagus and that only 1 cm of the proximal esophagus below the cricopharyngeal sphincter is entirely striated muscle.2 In contrast with the proximal portion of the cervical esophagus, the thoracic and abdominal esophagus and the lower esophageal sphincter (LES) consist entirely of smooth muscle and are composed of an inner circular and outer longitudinal layer. Throughout the length of the esophagus, there is no serosa overlying the muscle layers. The smooth muscle of the lower esophagus arises from the splanchnic mesenchyme and is supplied by nerves of the esophageal plexus derived from neural crest cells.1
Cervical Esophagus
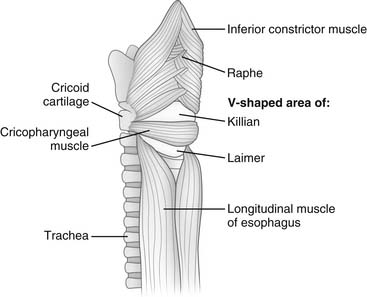
The cervical portion of the esophagus is about 5 cm long. It starts below the cricopharyngeus muscle and appears as a continuation of the inferior constrictor muscle of the pharynx. A space between the right and left inferior constrictor muscles posteriorly just above the cricopharyngeus muscle is called killian triangle and is the site where a Zenker’s diverticulum develops (Fig. 34-2). The beginning of the cervical esophagus is marked by the level of C6, and the end by the lower border of T1. The cervical esophagus curves slightly to the left as it descends. Anteriorly, it abuts the trachea and larynx and can be dissected off both organs. Posteriorly, the cervical esophagus lies on the vertebral bodies in a prevertebral or retroesophageal space. This space continues with a retropharyngeal space superiorly and is continuous with the posterior mediastinum. Laterally, the omohyoid muscle crosses the cervical esophagus obliquely, and it is usually divided to expose this portion of the esophagus. The carotid sheaths lie laterally, and the lobes of the thyroid and the strap muscles lie anteriorly. The recurrent laryngeal nerves lie in the grooves between the esophagus and the trachea. The right recurrent nerve runs a more lateral and oblique course to reach the groove and is more prone to anatomic variation. The surgical approach to the cervical esophagus may be from either side of the neck through an incision along the medial border of the sternocleidomastoid muscle. The left-sided approach is preferred to avoid injury to the right recurrent nerve.
Thoracic Esophagus
The thoracic portion of the esophagus is approximately 20 cm long (see Fig. 34-1) and starts at the thoracic inlet. In the upper portion of the thorax, it is closely related to the posterior wall of the trachea. This close relationship is responsible for the early spread of cancer of the upper esophagus into the trachea, and it may limit the surgeon’s ability to resect such a tumor. Above the level of the tracheal bifurcation, the esophagus courses to the right of the aortic arch and the descending aorta and then moves to the left, passing behind the tracheal bifurcation and the left main bronchus.
Abdominal Esophagus
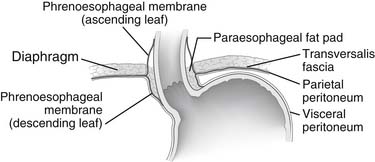
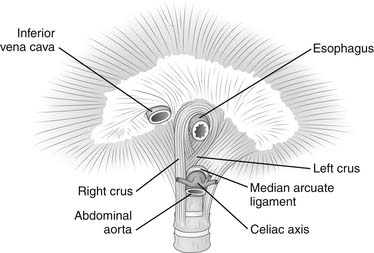
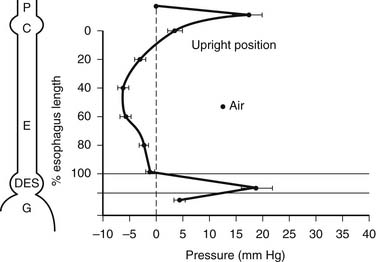
The abdominal portion of the esophagus is approximately 2 cm long and includes the abdominal portion of the LES. It begins as the esophagus passes through the diaphragmatic hiatus and is surrounded by the phrenoesophageal membrane, a fibroelastic ligament that arises from the subdiaphragmatic fascia as a continuation of the transversalis fascia lining the abdomen (Fig. 34-3). The upper leaf of the membrane attaches in a circumferential fashion around the esophagus about 1 to 2 cm above the level of the hiatus. The lower limit of the phrenoesophageal membrane blends with the serosa of the stomach, and its end is marked anteriorly by a prominent fat pad, which corresponds approximately with the gastroesophageal junction. The LES is a zone of high pressure 3 to 4 cm long at the lower end of the esophagus3 and does not correspond to any macroscopic anatomic change except for a slight thickening of the esophageal muscular wall. Its function is derived from the microscopic architecture of the muscle fibers. The esophageal hiatus is surrounded by the right and left crura, which together form a sling of skeletal muscle around the esophagus that originates from tendinous bands attached to the anterolateral surface of the first lumbar vertebra (Fig. 34-4). The relative contribution of the right and left crura to this sling is variable. Posterior to the esophagus, the crura are united by a tendinous arch—the median arcuate ligament—that lies just anterior to the aorta.
Blood Supply, Lymphatics, and Innervation
The cervical portion of the esophagus receives its main blood supply from the inferior thyroid artery. The thoracic portion receives blood from the bronchial and esophageal arteries. Seventy-five percent of individuals have one right-sided and two left-sided bronchial arteries, and usually two esophageal branches arise directly from the aorta. The blood supply of the abdominal portion of the esophagus comes from the ascending branch of the left gastric artery and from the right and left inferior phrenic arteries (Fig. 34-5). After the vessels have entered the muscular wall of the esophagus, branching occurs at right angles to provide an extensive longitudinal vascular plexus. The rich blood supply provided by this vascular plexus allows mobilization of the esophagus from the stomach to the aortic arch without causing ischemic injury.4
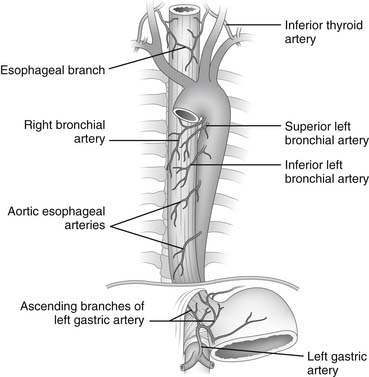
Figure 34–5 Arterial blood supply of the esophagus.
(Modified from Rothberg M, DeMeester TR. Surgical anatomy of the esophagus. In: Shields TW, editor. General thoracic surgery, 3rd ed. Philadelphia: Lea & Febiger; 1989. p. 84.)
The capillaries of the esophagus drain into a submucosal and periesophageal venous plexus, from which the esophageal veins originate. In the cervical region, the esophageal veins empty into the inferior thyroid vein; in the thoracic region, they empty into the bronchial, azygos, or hemiazygos veins; and in the abdominal region, they empty into the coronary vein (Fig. 34-6).
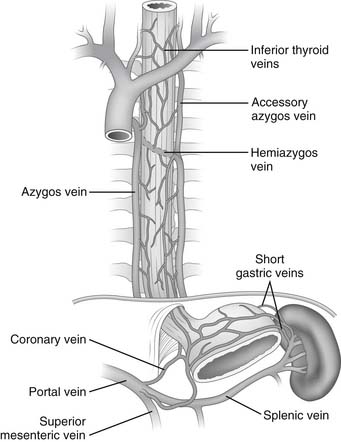
Figure 34–6 Venous drainage of the esophagus.
(Modified from Rothberg M, DeMeester TR. Surgical anatomy of the esophagus. In: Shields TW, editor. General thoracic surgery, 3rd ed. Philadelphia: Lea & Febiger; 1989. p. 85.)
The lymphatic channels are located almost exclusively below the muscularis mucosa in the submucosa of the esophagus. They are so dense and interconnected that they constitute a plexus (Fig. 34-7) with more lymph vessels than blood capillaries. Lymph flow in the submucosal plexus runs in a longitudinal direction, and after the injection of a contrast medium, the longitudinal spread is six times that of the transverse spread. In the upper two thirds of the esophagus, the lymphatic flow is mostly cephalad; in the lower third, it is mostly caudad. In the thoracic portion of the esophagus, the submucosal lymph plexus extends over a long distance in a longitudinal direction before penetrating the muscle layer to enter lymph vessels in the adventitia. As a consequence of this nonsegmental lymph drainage, the lymphatic spread of tumor cells can extend for a considerable distance superiorly and inferiorly within the submucosal lymphatics before the cells pass through lymphatic channels in the muscularis and on into the regional lymph nodes. By contrast, the cervical esophagus has a more segmental lymph drainage into the regional lymph nodes, and as a result, tumors in this portion of the esophagus have less submucosal extension.
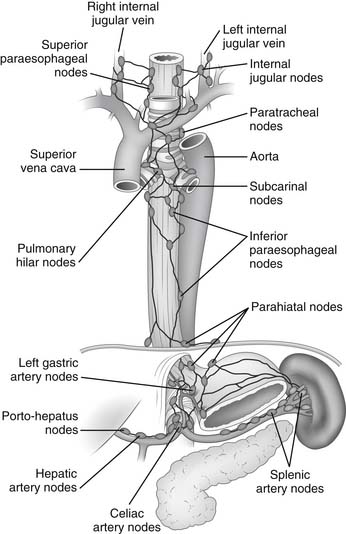
Figure 34–7 Lymphatic drainage of the esophagus.
(From DeMeester TR, Barlow AP. Surgery and current management for cancer of the esophagus and cardia: Part 1. Curr Probl Surg 1988;25:498.)
Lymph from the cervical esophagus drains into the paratracheal and deep cervical lymph nodes, whereas lymph from the upper thoracic esophagus flows mainly into the paratracheal lymph nodes. The lymph from the lower thoracic esophagus drains into the subcarinal and inferior pulmonary nodes. Lymph from the distal thoracic and abdominal portion of the esophagus drains into the parahiatal and left gastric nodes.1
The parasympathetic innervation of the pharynx and esophagus is provided mainly by cranial nerve X or the vagal nerve. The constrictor muscles of the pharynx receive branches from the pharyngeal plexus, which is located on the posterior lateral surface of the middle constrictor muscle and is formed by pharyngeal branches of the vagus nerve, with a small contribution from cranial nerves IX and XI. The cricopharyngeal sphincter and the cervical portion of the esophagus receive branches from both the right and left recurrent laryngeal nerves (Fig. 34-8). Damage to these recurrent nerves interferes not only with the movement of the vocal cords but also with the function of the cricopharyngeal sphincter and the motility of the cervical esophagus and predisposes the patient to pulmonary aspiration on swallowing. The upper thoracic esophagus receives innervation from the left recurrent laryngeal nerve and both vagal nerves. The esophageal plexus, which is formed by the branches of the right and left vagal nerves and thoracic sympathetic chain, lies on the anterior and posterior walls of the esophagus and innervates the lower thoracic portion.5 The branches of the plexus coalesce into the left (anterior) and right (posterior) vagal trunks.
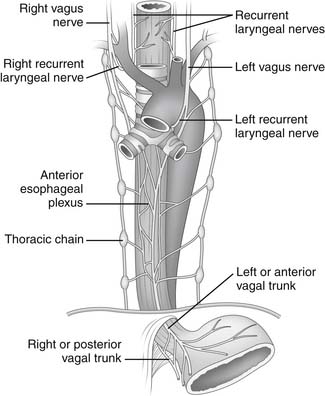
Figure 34–8 Innervation of the esophagus.
(Modified from Rothberg M, DeMeester TR. Surgical anatomy of the esophagus. In: Shields TW, editor. General thoracic surgery, 3rd ed. Philadelphia: Lea & Febiger; 1989. p. 85.)
PHYSIOLOGY
The Swallowing Mechanism
The act of alimentation requires the passage of food and drink from the mouth into the stomach. Food is taken into the mouth in a variety of bite sizes, after which it is broken up by the teeth, mixed with saliva, and lubricated. When food is ready for swallowing, the tongue, acting as a pump, moves the bolus into the posterior oropharynx and forces it into the hypopharynx (Fig. 34-9). Concomitantly with the posterior movement of the tongue, the soft palate is elevated, thereby closing the passage between the oropharynx and nasopharynx. With the initiation of the swallow, the hyoid moves superiorly and anteriorly, thereby elevating the larynx and enlarging the retropharyngeal space. At the same time, the epiglottis covers the laryngeal inlet to prevent aspiration.
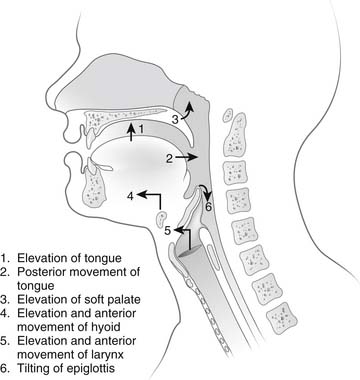
Figure 34–9 Sequence of events during the oropharyngeal phase of swallowing.
(From DeMeester TR, Stein HJ, Fuchs KH. Physiologic diagnostic studies. In: Zuidema GD, Orringer MB, editors. Shackelford’s surgery of the alimentary tract, 3rd ed, vol 1. Philadelphia: WB Saunders; 1991. p. 95.)
During swallowing, the pressure in the hypopharynx rises abruptly to 60 mm Hg as a result of the backward movement of the tongue and contraction of the posterior pharyngeal constrictors. A sizable pressure difference develops between the hypopharyngeal pressure and the subatmospheric midesophageal or intrathoracic pressure (Fig. 34-10). This pressure gradient speeds the movement of food from the hypopharynx into the esophagus when the cricopharyngeus or upper esophageal sphincter (UES) relaxes. The bolus is both propelled by the peristaltic contraction of the posterior pharyngeal constrictors and sucked into the thoracic esophagus by this pressure gradient.
Critical to receiving the bolus is the compliance of the cervical esophageal muscle and the timing and degree of relaxation of the UES. Abnormalities of compliance and UES opening result in pharyngeal dysphagia. During the transfer of the bolus from the mouth into the esophagus, the UES is mechanically pulled open. Elevation of the larynx by muscles attached to the hyoid bone pulls the UES open at the time muscle relaxation of the UES occurs. This is an active relaxation caused by a reduction in the tone of the tonic cricopharyngeus muscle, and it is dependent on a neurologically mediated reflex. This is an all-or-nothing event; partial relaxation does not normally occur. The UES closes within 0.5 second of the initiation of the swallow, with a postrelaxation contraction pressure that is approximately twice the resting pressure of 30 mm Hg. The postrelaxation contraction continues down the esophagus as a peristaltic wave (Fig. 34-11). The high closing pressure and the initiation of the peristaltic wave prevent reflux of the bolus from the esophagus into the pharynx. After completion of the swallow, the pressure of the UES returns to its normal resting pressure.
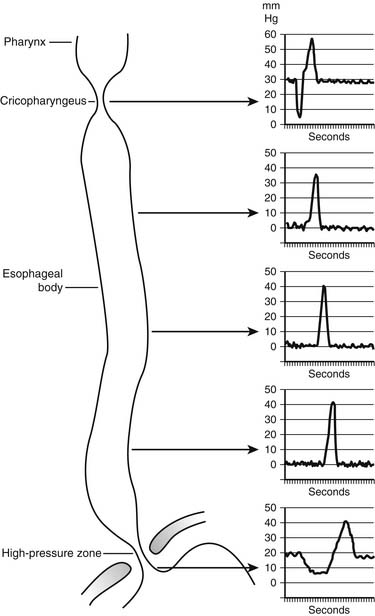
Figure 34–11 Intraluminal esophageal pressures in response to swallowing.
(From Waters PF, DeMeester TR. Foregut motor disorders and their surgical management. Med Clin North Am 1981;65:1237.)
The peristaltic wave generates an occlusive pressure that varies from 30 to 120 mm Hg. The wave rises to a peak in 1 second, remains at the peak for about 0.5 second, and then subsides in about 1.5 seconds. The whole course of the rise and fall of an occlusive contraction may occupy one point in the esophagus for 3 to 5 seconds.6,7 The peak of the primary peristaltic contraction moves down the esophagus at a rate of 2 to 4 cm per second and reaches the distal esophagus about 9 seconds after swallowing starts. A consecutive swallow in 20 seconds produces a similar primary peristaltic wave; however, if the swallow occurs sooner, the esophagus is inhibited and unresponsive.
Lower Esophageal Sphincter
The LES has no anatomic landmarks, but its presence can be identified by a rise in pressure over gastric baseline pressure when a pressure transducer is pulled from the stomach into the esophagus. This high-pressure zone is normally present except in two situations: (1) after a swallow, when it is momentarily dissipated or relaxes to allow passage of food into the stomach; and (2) during a belch, when it allows gas to be vented from a distended fundus. The common denominator for virtually all episodes of gastroesophageal reflux is the loss of this normal high-pressure zone or barrier. When the barrier is absent, resistance to the flow of gastric juice from an environment of higher pressure (the stomach) to an environment of lower pressure (the esophagus) is lost. In early GERD, this is usually caused by a transient loss of the barrier. In advanced GERD, there is usually a permanent loss of the barrier.8
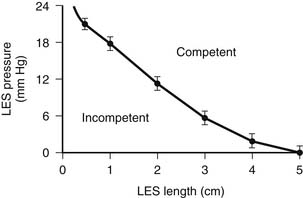
There are three characteristics of the LES or high-pressure zone that maintain its function as a barrier to intragastric and intra-abdominal pressure challenges. Two of these characteristics—the overall length and pressure of the LES—work together and depend on each other to provide resistance to the flow of gastric juice from the stomach into the esophagus.9 The shorter the overall length, the higher the pressure must be for the LES to maintain sufficient resistance to remain competent (Fig. 34-12). Consequently, the effect of a normal LES pressure can be nullified by a short overall LES length, and the effect of a normal overall LES length can be nullified by a low LES pressure. For practical purposes, the pressure of the LES is measured at a single point, but in actuality, pressure is applied over the entire length of the LES; this allows the computer formation of a three-dimensional image of the LES or barrier (Fig. 34-13). The volume of this image reflects the resistance of the LES to the flow of fluid through it, which is called the sphincter pressure vector volume. A calculated volume below that of the fifth percentile of normal resting subjects indicates a permanently defective LES.10 A fundamental principle for surgeons to understand is that the length of the barrier or LES is critical to its function. Shortening of LES length occurs naturally with gastric filling as the terminal esophagus is “taken up” by the expanding fundus (Fig. 34-14)11; this is similar to the shortening of the neck of a balloon as it is inflated. With excessive gastric distention (e.g., with overeating), the length of the LES shortens to a critical point at which it gives way, the pressure drops precipitously, and reflux occurs (Fig. 34-15).12 If the length of the LES is permanently shortened, then further shortening caused by the normal gastric distention with normal-volume meals results in postprandial reflux. In this situation, competency of the barrier is an ever-constant clinical problem. The observation that gastric distention results in shortening of the LES down to a critical length so that the pressure dissipates, the lumen opens, and reflux occurs provides a mechanical explanation for transient LES relaxations without invoking a neuromuscular reflex. If only the LES pressure and not its length is measured (e.g., with a Dent sleeve), the event appears as a spontaneous relaxation of LES pressure.13 In reality, it is the progressive shortening of the LES rather than the transient LES relaxations that results in the loss of LES pressure.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree