Chapter 4 Endovenous Thermal Ablation of Saphenous Reflux
Historical Background
The exact historical point in time when saphenous vein incompetence was recognized as a source of venous hypertension is unclear; however, Trendelenburg promulgated saphenofemoral ligation in 1891.1 In the early 20th century, stripping of the saphenous veins was added to proximal ligation. Keller2 described an internal stripper in 1905. Hence, high ligation of the great saphenous vein (GSV) at the saphenofemoral junction (SFJ) followed by GSV stripping from groin to knee, or ankle was the standard of care and was performed in the hospital setting for about 100 years.
Etiology and Natural History of Disease
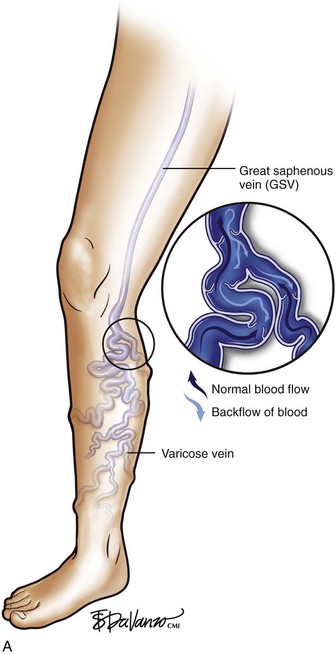
The majority of patients (60% to 70%) with varicose veins have an incompetent SFJ and GSV reflux.3 It is critical to recognize that bulging varicose veins are usually associated with an underlying source of venous hypertension, and treatment of the source is as important as is treatment of the actual varicose vein.
Chronic venous disorders generally result from primary venous insufficiency or secondary processes, such as acute deep venous thrombosis (DVT) or trauma. An analysis of chronic venous disease indicated that primary valvular incompetence was present in 70% to 80% of cases; secondary valvular incompetence was due to trauma or DVT in 18% to 25%, and congenital anomaly was present in 1% to 3%.4
Patient Selection
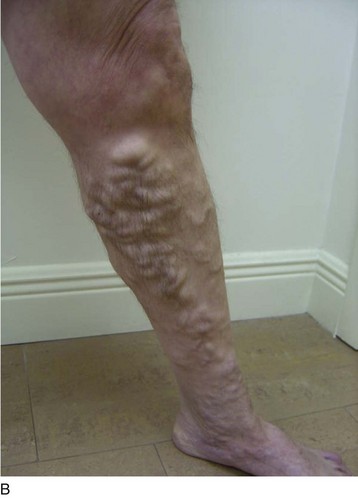
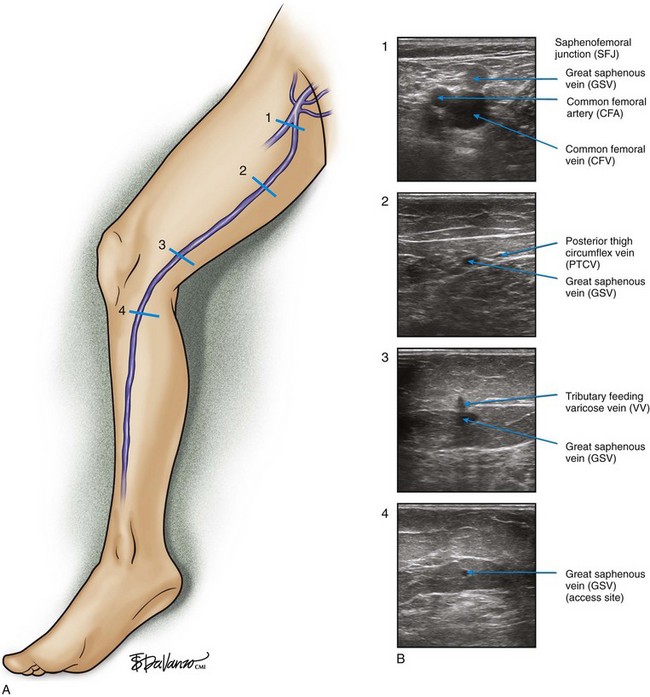
As is customary for any medical condition, the physician must begin with a careful history and physical examination. The primary purpose of the clinical examination of the patient presenting with chronic venous disease (CVD) is to classify the subject using the popular CEAP system5,6—clinical (telangiectasias to skin damage), etiologic (primary, secondary, or congenital), anatomic (superficial, deep, or perforators), and pathophysiologic (reflux, obstruction, or both) patterns, forming the acronym CEAP. For each of these major classifications, there are subgroups. For the work described in this chapter, clinical signs emerge as the most important and are grouped as follows—C1: spider telangiectasias; C2, varicose veins (Fig. 4-1); C3, edema; C4, lipodermatosclerosis; C5, healed ulcer; and C6, active ulcer. Regarding treatment, the class (C) is the most important parameter to establish during the initial encounter. Treatment algorithms for chronic venous insufficiency (CVI) (i.e., patients with more severe disease [C4, C5, C6]) are discussed in other sections of this book. This chapter focuses on the treatment of C2 disease.
Endovascular Instrumentation
Device choice is a matter of physician preference. Our center and other investigators have compared the efficacy of RF and EVL. The ablation data are slightly better for EVL.7,8 A few years ago, we published our 3-year data showing 94% success with RF and 98% success with laser9; however, in current practice, the results are closer to 98% success with either technology.
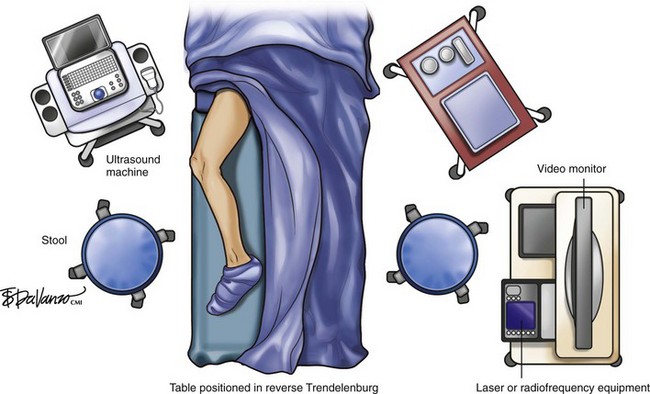
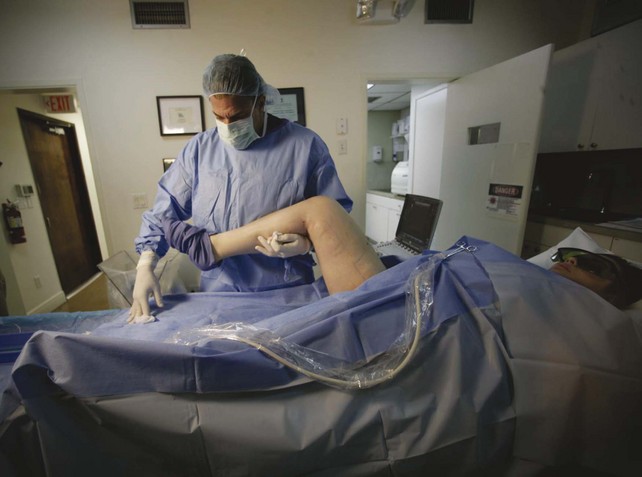
Figure 4-2 depicts the general layout of an office-based venous surgery suite. An operating table with a back table is prepared in the usual sterile manner. The laptop ultrasound system is mounted on a movable cart, and the thermal ablation equipment is in close proximity to allow easy viewing of the display panels by the operator. Hemodynamic monitoring equipment (heart rate, blood pressure, oxygen saturation) is available and is used during cases offering conscious sedation. If local anesthesia without sedation is used, hemodynamic monitoring is not required in most states.
Endovascular Laser
Imaging
Access and Closure
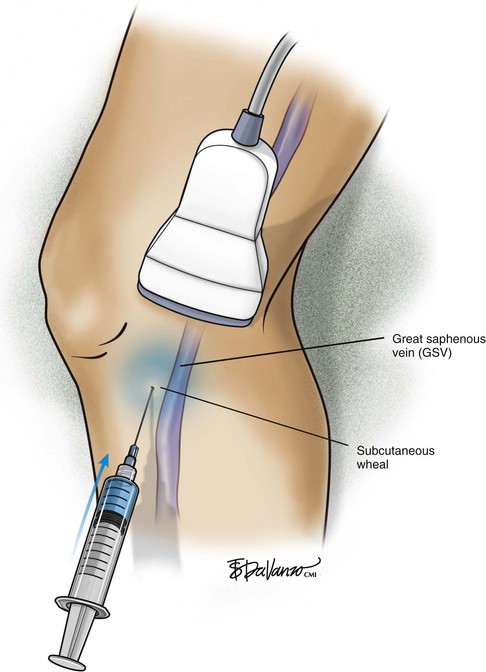
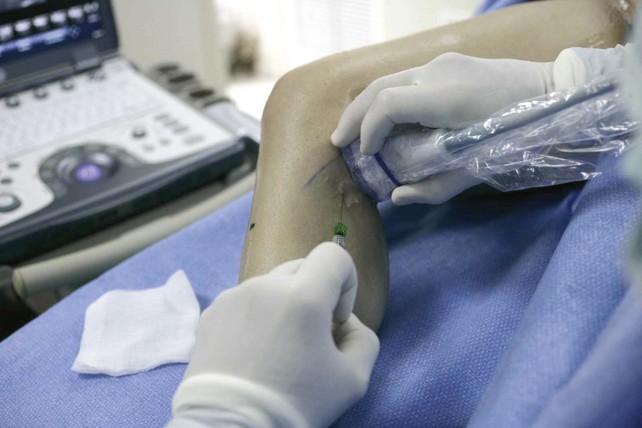
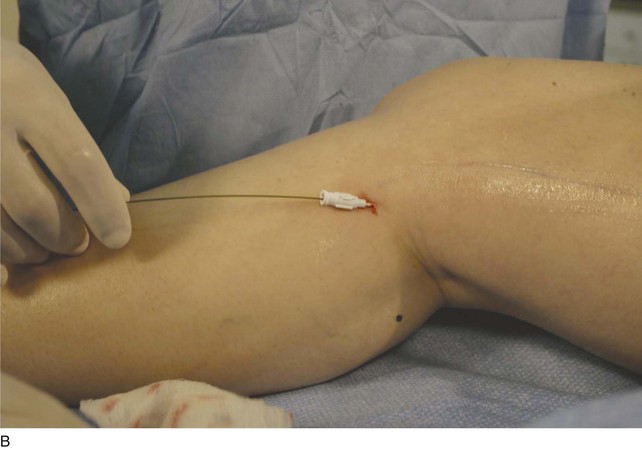
The surgeon begins by placing a wheal of local anesthesia on the skin access site with a syringe and small (25- to 30-gauge) needle (Fig. 4-4). The access needle is then held at an approximately 45-degree angle 1 inch from the ultrasound probe, and the target vein will be located at the tip of an imaginary triangle where the ultrasound beam and the tip of the access needle meet under the skin. For large veins (>5-mm diameter), an 18-gauge needle is used. For smaller diameter veins (<5-mm diameter), a 21-gauge needle and micropuncture assembly are preferred (Fig. 4-5).
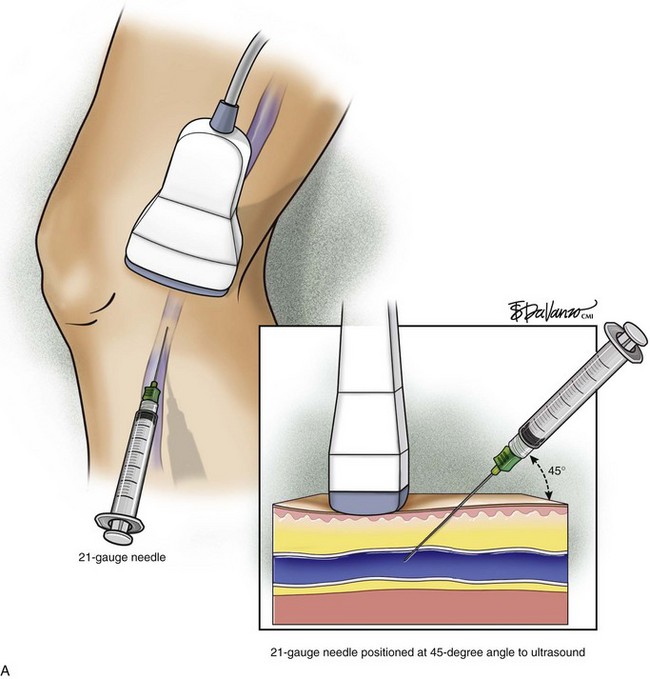
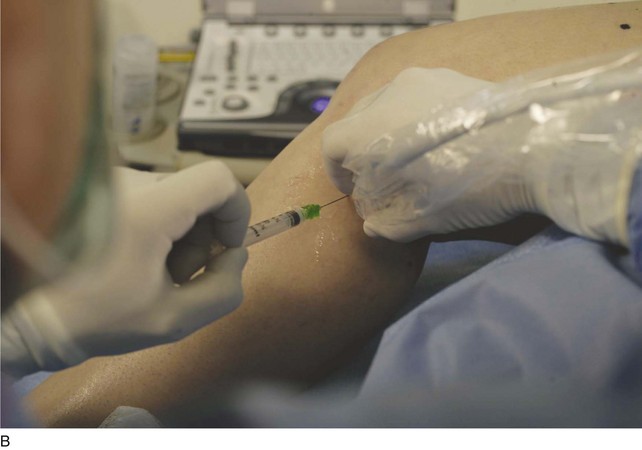
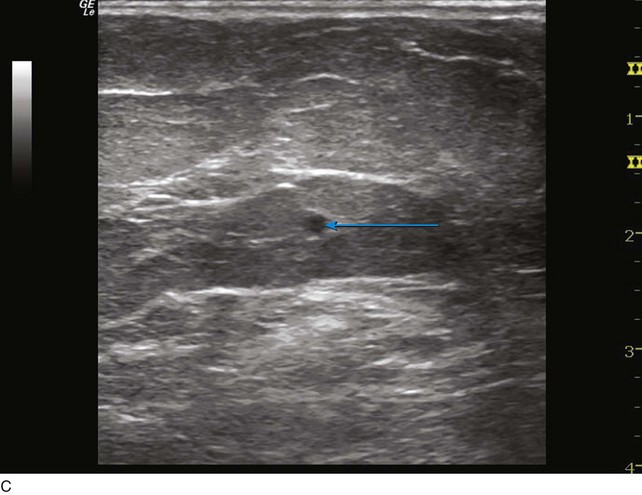
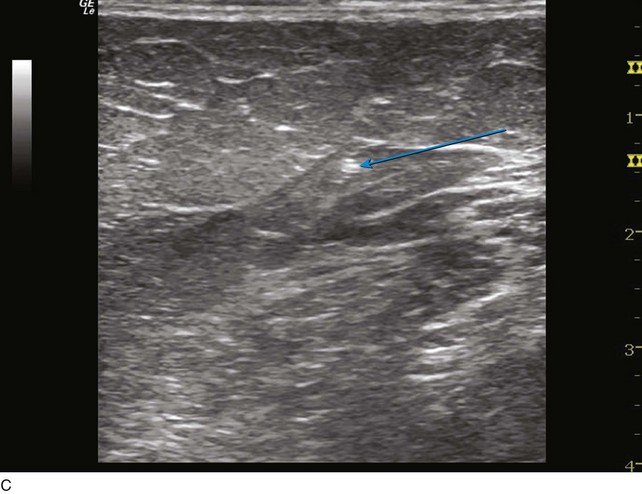
Fig 4–5 C, Cross-sectional ultrasound image of great saphenous vein (GSV) (the target vein) (arrow).
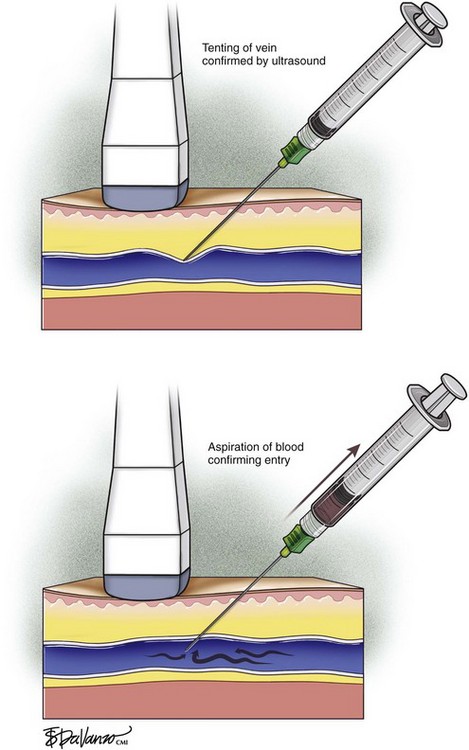
The needle tip is guided to the roof of the vein using ultrasound to visualize where the puncture will take place. Usually one will tent the roof of the vein with a gentle push, easily seen with ultrasound imaging, prior to a more forceful motion for entry. Aspiration of dark nonpulsatile blood into a connected syringe confirms venous entry (Fig. 4-6).
Hemostasis and Anticoagulation
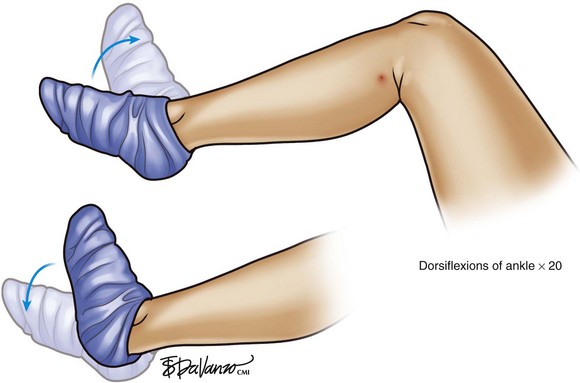
As demonstrated in Figure 4-7, we believe that on-table activation of the calf pump is very effective in preventing thromboembolic complications during thermal ablation procedures. After the ablation and before the ambulatory phlebectomy portion of the procedure (discussed in Chapter 9), we simply ask the patient to actively dorsiflex and plantarflex the foot 20 times.
Operative Steps
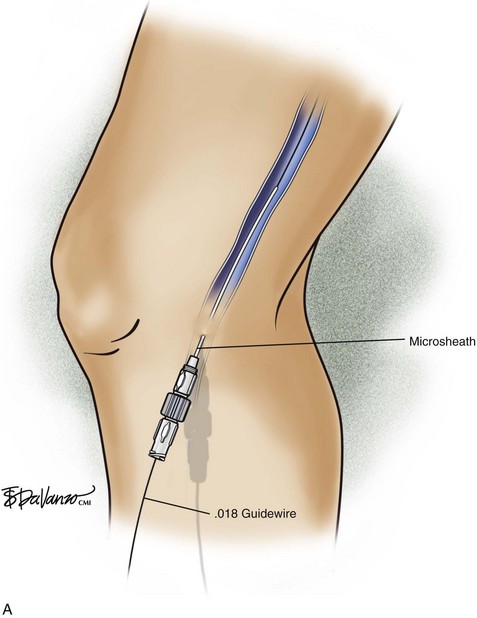
The ultrasound probe is held perpendicular to the skin to demonstrate the target vein in either the short or long access on the ultrasound screen. Once venous entry is confirmed, a guidewire is chosen. The micropuncture needle accepts a .018-inch wire followed by a 4-Fr coaxial microsheath and its intraluminal dilator. Usually a small stab incision with a No. 11 blade scalpel is required at the wire entry site to widen the incision for the microsheath. Confirmation that the .018-inch wire is in the endoluminal space is performed with ultrasound (Fig. 4-8).
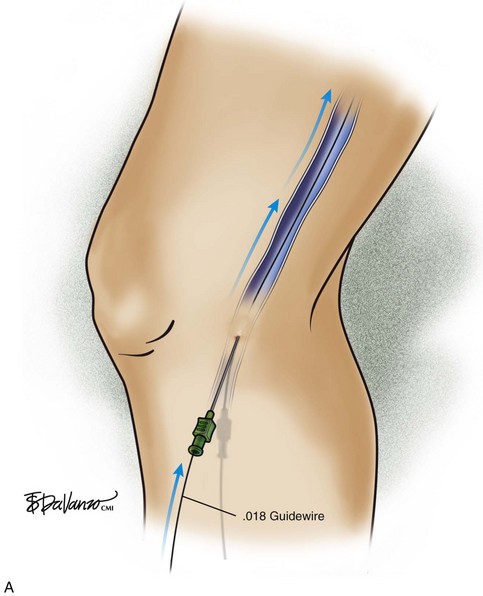
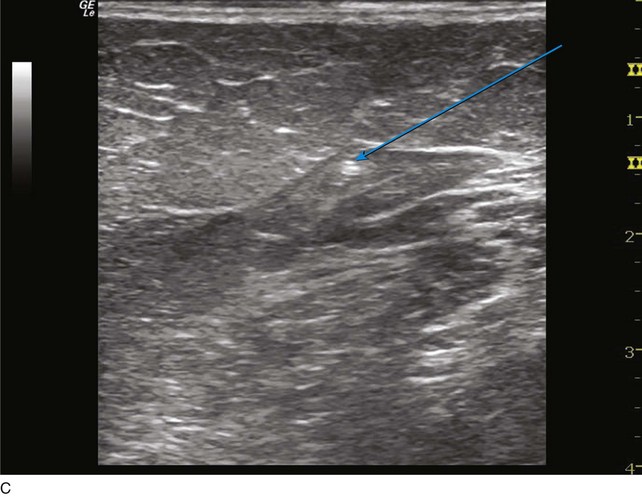
Fig 4–8 C, Cross-sectional ultrasound image demonstrating intraluminal placement of .018 guidewire (arrow).
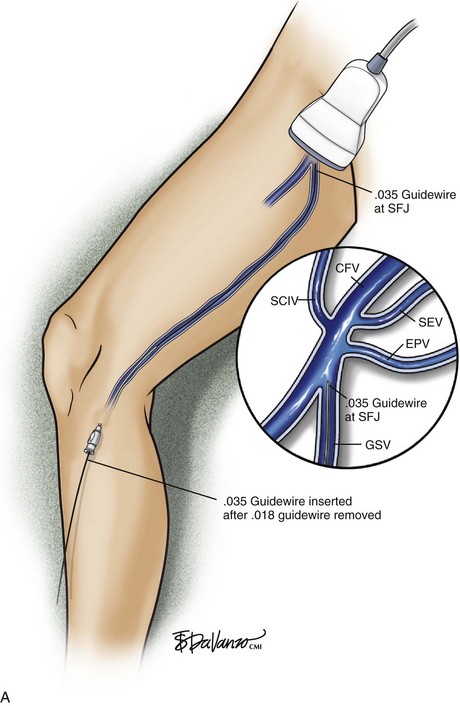
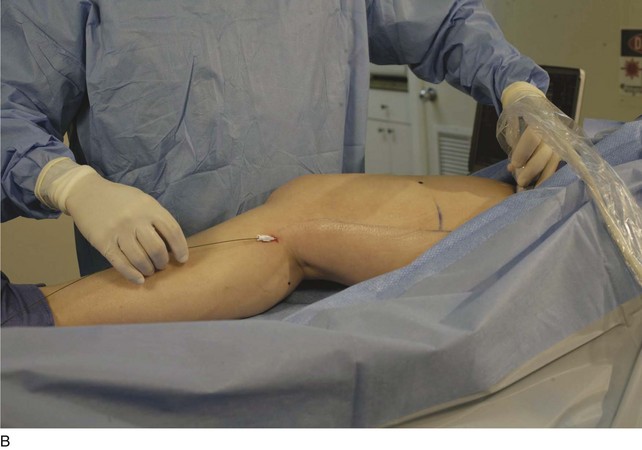
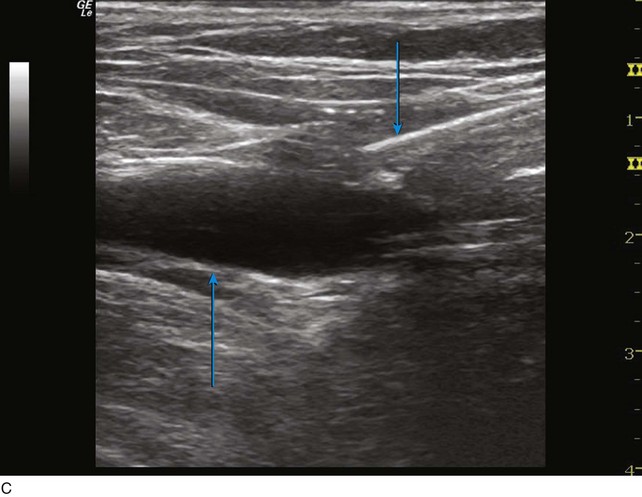
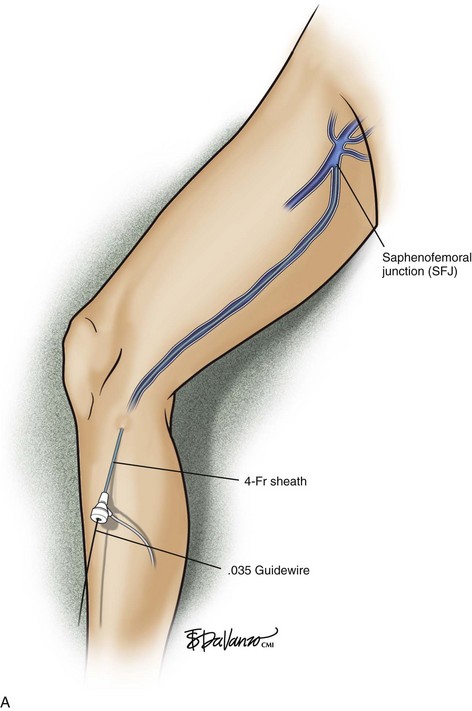
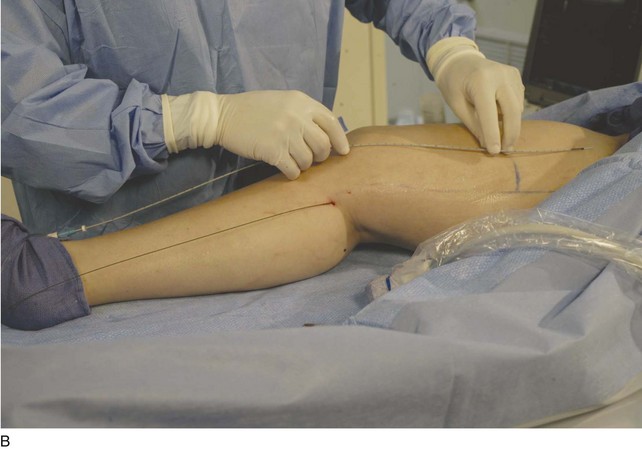
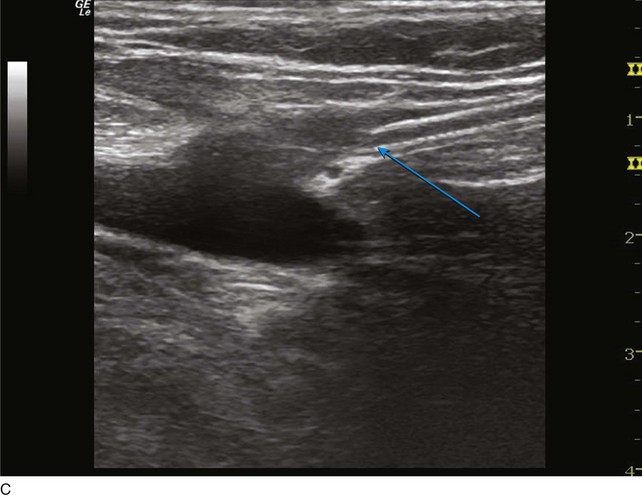
The microsheath assembly is placed, the microdilator and .018-inch guidewire are removed, and the remaining microsheath is used to place a .035-inch guidewire (Fig. 4-9, A). The .035-inch guidewire is then navigated to the SFJ using ultrasound control (Fig. 4-9, B and C).
Some operators prefer to deliver the wire with the J-tip at the lead, but we prefer to send the straight end of the wire up first, especially in smaller veins. J-tipped wires may cause distention of the vein and induce friction with the inner lining of the vein wall during passage. This generally will cause pain secondary to venous distention, which activates the adrenergic sympathetic nerve fibers residing in the adventitia (Fig. 4-10).
Once the tip of the wire is positioned at the SFJ, a larger coaxial sheath is placed (Fig. 4-11).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree