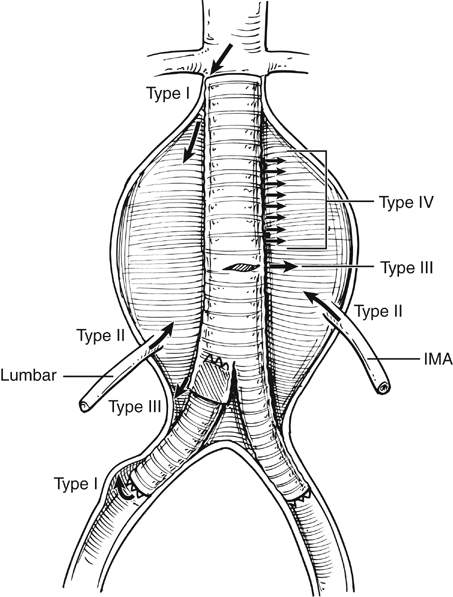
Endovascular aneurysm repair (EVAR) has become established as the first-line approach to treating abdominal aortic aneurysms (AAAs). Conceptually, the idea of using vascular endoprosthesis to exclude aneurysms dates back to the late 1960s with animal experimentation. The landmark first deployment of an aortic stent to exclude a human AAA was reported by Parodi and colleagues in 1991. Initially, straight grafts were used, with polyester tubes being held in position within the aorta by large Palmaz stents. It was soon realized that as few as 5% of infrarenal AAAs had an anatomy suitable for such a device, resulting in a high incidence of failure as a result of type I endoleaks (Figure 1) With time, modular bifurcated grafts became the preferred option for infrarenal AAA repair.
Endovascular Treatment of Nonruptured Infrarenal Aortic and Aortoiliac Aneurysms
Thoracic Key
Fastest Thoracic Insight Engine