The same principles used to treat atherosclerotic carotid bifurcation lesions are applied to FMD lesions, with a few important modifications. Pharmacologic prevention of cerebrovascular events starts before the procedure with oral aspirin and clopidogrel. Dual antiplatelet therapy is continued for 4 to 6 weeks, followed by aspirin alone, except for patients treated by covered stents who receive dual antiplatelet regimen indefinitely.
The procedure is usually performed using the transfemoral approach, with monitored anesthesia care. Intravenous heparin (100 units/kg) is administered to achieve a target activated clotting time of longer than 250 seconds before any catheters are manipulated in the aortic arch. Any abnormal or possibly abnormal arterial segments of the carotid, vertebral, and cerebrovascular anatomy identified by computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) should be evaluated with angiography.
In patients with type I arch, a simple catheter (e.g., JB1, vertebral) is preferred; curved catheters (e.g., Simmons II, Vitek) are used in patients with difficult arches (types II and III). Selective catheterization of the mid-distal common carotid artery (CCA) is performed over a stiff 0.035-inch angled glide wire using the Shuttle select system. If flow reversal, balloon occlusion, or a larger (>7 Fr) sheath is used, advancement of the sheath requires placement of an exchange length 0.035-inch stiff glide wire into the terminal branches of the external carotid artery. It is important to maintain sheath access to the CCA, realizing that once the 0.035-inch stiff wire and dilator are removed, support is provided solely by the sheath.
Fibromuscular dysplasia lesions are by nature more prone to dissection, are longer than atherosclerotic lesions, can harbor small debris, and often extend to the distal ICA. Filter wires are typically not very steerable to navigate the tortuous membranous segments of FMD. In addition, the filter needs to be deployed within an uninvolved straight segment of cervical ICA, which is not always available. In these cases the use of flow reversal or balloon occlusion can decrease risk of embolization, which can occur during unprotected guidewire manipulations. It is preferable to cross the lesion with a soft 0.018-inch glide wire and microcatheter.
After the lesion is crossed and true lumen access is confirmed, the interventional 0.014-inch guidewire of choice is positioned. The Gore Flow Reversal system (WL Gore, Flagstaff, AZ) has a 6-Fr working sheath compatible with most small-profile, rapid-exchange angioplasty balloons and self-expandable stents. A small-profile (0.018-inch) 5- or 6-mm Gore Viabahn stent graft (WL Gore, Flagstaff, AZ) may be used over a 6-Fr sheath to treat aneurysms or FMD lesions that have large outpouchings. If a larger stent graft (7 or 8 mm) is needed, the sheath size must be increased to 7 Fr. In these cases, the technique is modified and a 0.014-inch filter wire with long (>300 cm) working length is used. A larger delivery system can require direct surgical exposure of the carotid arteries, which allows flow reversal by clamping the CCA and external carotid artery.
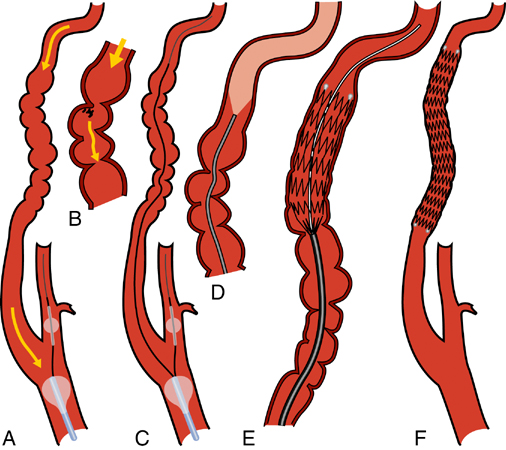
Performing angioplasty alone or in conjunction with a stent or stent graft is somewhat controversial given the small number of patients reported in the literature. Although primary angioplasty has been shown to be safe and durable for renal FMD, it leaves multiple small intimal flaps, membranes, and debris that pose an ongoing risk of embolization during or after carotid interventions. Our preference is to use self-expanding stents (Figure 1) to scaffold the affected ICA segment, unless this cannot be done safely because of tortuosity or other anatomic constraints. For aneurysms or larger outpouches with beaded dilatations that may contain embolic material, low profile stent-grafts are used (Figure 2). For excessively tortuous or focal lesions, primary angioplasty is used. Administration of atropine (0.5–1 mg) may be considered for de novo lesions as prophylaxis against bradycardia during balloon inflation in the carotid artery.
Only gold members can continue reading.
Log In or
Register to continue
Related
![]()



