Endovascular Treatment of Descending Thoracic Aortic Aneurysms
Darren B. Schneider
Endovascular thoracic aortic aneurysm repair is an emerging therapeutic alternative to traditional open surgical repair that involves intraluminal insertion of a stent graft through a remote transvascular (femoral, iliac, or aortic) approach to exclude the aneurysm from the circulation. The endovascular approach is attractive by virtue of its potential to avoid the high morbidity and mortality associated with standard open surgical repair due to thoracic or thoracoabdominal exposures and the systemic consequences of transient aortic occlusion and visceral/renal ischemia. Following the recent FDA approval of the first stent graft for treatment of thoracic aortic aneurysms in the United States, endovascular thoracic aortic aneurysm repair is rapidly becoming the preferred modality for treating thoracic aortic aneurysms in many centers. While conceptually a straightforward procedure involving insertion of a cylindrical stent graft into the aorta, a variety of anatomic and physiologic factors can render endovascular thoracic aortic aneurysm repair challenging in actual clinical practice. The complexity of endovascular thoracic aortic repair is increased by aortic tortuosity, proximity of the aneurysm to brachiocephalic or visceral aortic branches, narrow or tortuous iliac access arteries, and risk of devastating complications, such as embolic stroke and spinal cord ischemia. Consequently, the successful endovascular repair of thoracic aortic aneurysms requires high-quality preprocedural imaging, careful patient selection and preprocedural planning, and technical skill in endovascular procedures.
Presentation, Natural History, and Diagnosis
The majority of patients with thoracic aortic aneurysms are asymptomatic, and most aneurysms are discovered incidentally on routine imaging studies obtained during evaluation of another disorder. When thoracic aortic aneurysms cause symptoms, patients most often report chest or back pain, which may be a sign of impending rupture. Occasionally, thoracic aortic aneurysms may present with symptoms related to local mass effect or erosion. Compression of the trachea or mainstem bronchus may produce respiratory compromise; esophageal compression may produce dysphagia; and compression of the recurrent laryngeal nerve can produce vocal cord paralysis and hoarseness. Hemoptysis is rare, but it may indicate an aorto-bronchial fistula due to erosion into a tracheobronchial structure. Rupture, as the initial clinical presentation, is nearly always fatal, and the majority of patients succumb before arriving at a hospital.
With the decline in the incidence of syphilitic aneurysms, thoracic aortic aneurysms are now much less prevalent than abdominal aortic aneurysms; an estimated annual incidence is 6 cases per 100,000 persons. The descending thoracic aorta is involved in approximately 40% of patients, and the aortic arch is involved in approximately 10% of patients with thoracic aortic aneurysms. Descending thoracic aortic aneurysms are principally associated with medial degeneration and luminal atherosclerosis; less common causes include trauma, infection, pseudoaneurysms after prior aortic grafting, and connective tissue disorders, such as Marfan syndrome or Ehlers-Danlos syndrome. Chronic aortic dissections are also prone to aortic dilation and aneurysmal degeneration over time, mandating vigilant surveillance in this patient population.
The natural history of thoracic aortic aneurysms remains poorly defined; however, up to 70% of patients with a thoracic aortic aneurysm that are followed without intervention will progress to aneurysm rupture, and more than 90% of ruptures are fatal. On the basis of longitudinal data, thoracic aortic aneurysms have an average annual growth rate of approximately 0.1 cm per year, but there is substantial interpatient variability, and growth rates may not be predicted with any certainty in clinical practice. For fusiform aneurysms, the annual risk of rupture is related to maximum aneurysm diameter and has been estimated to be 2% for aneurysms <5 cm in diameter, 3% for aneurysms between 5 cm and 6 cm, and 7% for aneurysms in excess of 6 cm. Even less is known about the natural history of penetrating aortic ulcers and saccular aneurysms. While still controversial, there is some consensus that repair should be considered for symptomatic or large penetrating ulcers and pseudoaneurysms regardless of size.
The presence of a thoracic aortic aneurysm may be suspected on the basis of a chest radiograph that shows an enlarged aortic silhouette. Contrast-enhanced CT is the preferred modality to define accurately aortic anatomy and measure accurately aortic aneurysm size, especially with current multislice scanners and the application of multiplanar
reconstruction software. MR angiography can also be useful, particularly in patients with impaired renal function. Transthoracic echocardiography fails to provide adequate visualization of the entire descending thoracic aorta. While transesophageal echocardiography does provide visualization of the entire thoracic aorta, it is invasive and operator-dependent, and its utility is largely limited to patients with acute aortic dissection. Conventional aortography as a diagnostic modality has been largely supplanted by cross-sectional imaging with CT angiography.
reconstruction software. MR angiography can also be useful, particularly in patients with impaired renal function. Transthoracic echocardiography fails to provide adequate visualization of the entire descending thoracic aorta. While transesophageal echocardiography does provide visualization of the entire thoracic aorta, it is invasive and operator-dependent, and its utility is largely limited to patients with acute aortic dissection. Conventional aortography as a diagnostic modality has been largely supplanted by cross-sectional imaging with CT angiography.
Indications and Contraindications
Most surgeons advocate repair of aneurysms with a diameter of 6 cm or greater. Patients with Marfan disease or a chronic aortic dissection may be at increased risk for rupture, and the threshold for repair is often reduced to 5 cm in these patient populations. Presence of symptoms or documented rapid enlargement are also indications for aneurysm repair for aneurysms less than 6 cm in diameter. Asymptomatic aneurysms less than 6 cm may be observed with surveillance CT or MR scans performed at 6-to 12-month intervals.
Because endovascular repair of thoracic aortic aneurysms is a relatively new and evolving procedure, only short- and mid-term outcome data are available. While early outcomes compare favorably with open surgery, more long-term data must be acquired before endovascular thoracic aortic repair is recommended as the preferred treatment approach for all patients with thoracic aortic aneurysms. Accordingly, endovascular repair should currently be reserved for patients at high risk for open surgical repair due to underlying cardiopulmonary disease or advanced age. Application of endovascular repair is also limited by anatomic constraints (covered in the “Patient Selection and Preprocedural Planning” section of this chapter). Young, good-risk patients may be more appropriately served by open surgical repair, which has proven durability.
Treatment of thoracic aortic aneurysms in the setting of chronic aortic dissection also merits further discussion. Aortic dissection significantly increases the complexity of endovascular approaches by introducing additional hemodynamic and anatomic factors. Compromise or compression of the true lumen, the presence of multiple intima tears, and differential perfusion of visceral branches from the true and false lumens may increase the risk of endoleak and branch vessel hypoperfusion after endovascular repair. Currently available stent graft devices are not specifically designed for treating chronic aortic dissections. Results of endovascular treatment of thoracic aortic aneurysms in the context of chronic aortic dissection is limited to case reports and small single-institution series. Accordingly, caution is advocated when considering endovascular repair in patients with aneurysmal dilatation of extensive chronic aortic dissections, and only patients without a viable open surgical option should be considered for endovascular repair until additional supportive data becomes available.
Likewise, connective tissue disorders represent a relative contraindication to endovascular repair. Patients with generalized compromise of arterial strength may be at increased risk for device-related complications, such as aortic dissection, perforation, and device migration.
Patient Selection and Preprocedural Planning
Proper patient selection and preprocedural planning are just as important as surgical technique for successful endovascular repair of thoracic aortic aneurysms. Risk stratification with assessment of cardiac and pulmonary function assist with the decision of whether to proceed with either open surgical or endovascular repair. Endovascular repair and subsequent follow-up imaging also require repeated contrast administration, so baseline renal function should be evaluated.
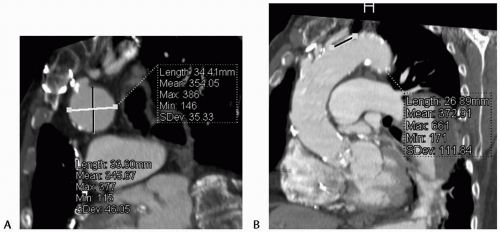
Detailed anatomic information is obtained using CT angiography with an axial slice thickness of less than 2 mm. Multiplanar reconstructions are essential to obtaining accurate aortic diameter and length measurements (Fig. 12-1). Reconstructions also allow assessment of tortuosity and angulation. Scans should extend from the aortic arch branches to the common femoral arteries for evaluation of the treatment area as well as the arterial access route. CT provides additional information about vessel calcification and mural thrombus, making it the preferred preprocedure imaging modality.
Evaluation and planning begins with assessing the proximal and distal necks for device fixation and seal to determine anatomic suitability for endovascular repair. The largest currently available stent graft has a diameter of 44 mm; therefore, the diameter of the neck of the aneurysm should not exceed 40 mm, factoring in minimum acceptable device oversizing. Neck length requirements for endovascular repair are device specific. In general, the neck length must be a minimum of 2 cm to obtain adequate fixation and seal.
Secure fixation and sealing of stent graft devices is also dependent on neck morphology and may be compromised by curvature or angulation within the neck. The neck should be relatively free of angulation or significant curvature, which may prevent apposition of the stent graft to the aortic wall throughout the neck zone and produce a perigraft Type I endoleak. Tortuosity also makes precise deployment of the stent graft difficult, which must be taken into consideration when planning treatment of an aneurysm with a short proximal neck. In general, it is better to cover a greater length of aorta to achieve secure fixation
and sealing than to sacrifice neck quality in an effort to minimize aortic coverage.
and sealing than to sacrifice neck quality in an effort to minimize aortic coverage.
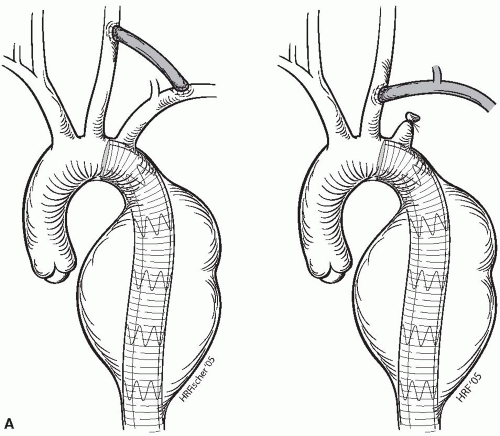
If necessary, coverage of the left subclavian artery may be planned to gain additional proximal neck length. Acute occlusion of the left subclavian artery by a stent graft has been well tolerated, and routine carotid subclavian bypass or subclavian transposition is necessary only rarely. Symptoms are infrequent, usually presenting with left upper-extremity effort fatigue and not with limb-threatening ischemia. Before covering the left subclavian artery, however, it is essential to know if the patient has a patent left internal mammary aortocoronary bypass and also to exclude the presence of significant occlusive disease of the contralateral left vertebral artery. Patency and antegrade flow within the contralateral vertebral artery is assessed by duplex ultrasonography, MR angiography, or conventional angiography. In cases of a patent left mammary artery graft or contralateral vertebral artery disease, a left subclavian artery transposition or carotid-subclavian bypass is performed prior to stent graft deployment (Fig. 12-2A).
Coverage of both the left common carotid and the left subclavian arteries to secure adequate proximal neck length may be done after creation of a carotid-carotid bypass (Fig. 12-2B). Right-to-left carotid-carotid bypass is performed using a reinforced 8 mm diameter PTFE graft placed through the retropharyngeal space. Coverage of the entire aortic arch is also possible if preceded by placement of antegrade bypasses from the ascending aorta to the great vessels; however, experience with such approaches is limited (Fig. 12-2C).
Similar anatomic criteria are used to assess suitability of the distal aortic neck for endovascular repair. Coverage of a patent celiac artery may result in acute visceral ischemia and should be avoided. Creation of retrograde visceral bypasses may be performed prior to stent graft deployment to allow more distal extension of the stent graft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree