Endovascular Treatment for Pelvic Congestion Syndrome
Carl M. Black
Ellen D. Dillavou
Symptoms and Indications for Intervention
Chronic pelvic pain is potentially debilitating and affects millions of women worldwide with a reported incidence of up to 39%. Routine evaluation yields no obvious etiology for chronic pelvic pain in up to one-third of cases. In cases in whom there is no identifiable cause of pelvic pain, an estimated 30% may actually have pelvic venous congestion (PVC)—also known as pelvic venous insufficiency (PVI) or pelvic congestion syndrome (PCS). Unfortunately, PVC is still often overlooked in the differential diagnosis of chronic pelvic pain.
Affected patients are generally multiparous and typically present in their late 20s or early 30s with a history of chronic pelvic pain of greater than 6 months’ duration. The classic and almost pathognomonic presentation includes varying degrees of positional pelvic and lower back pain that is exacerbated by prolonged standing and strenuous activity. Other symptoms include the following.
Pain is often described as heaviness and fullness in the lower pelvis, vulvar region, and thighs.
Pain is typically worse with menses.
Pain may be associated with dyspareunia and prolonged postcoital discomfort.
Pain is generally most severe at the end of the day.
Pain is frequently diminished with supine positioning.
Patients often experience the most relief upon awakening in the morning.
The combination of postcoital ache and ovarian point tenderness is reported to be 94% sensitive and 77% specific for the diagnosis of PVC when confirmed by venography. Patients may present with atypical nonsaphenous pudendal, vulvar, and perilabial varicosities which are visible on physical examination and ultrasound (US). Incompetent pelvic varices also often collateralize into posterolateral thigh and gluteal regions. An increased incidence of symptomatic PVC has been reported in patients suffering from complex nonsaphenous patterns of lower extremity superficial venous insufficiency.
The discomfort of PVC is due to venous distension of ovarian and pelvic varicosities. It is associated with uterine enlargement, thickened endometrium, and cystic changes in the ovaries. The etiology of PVC is multifactorial, and includes factors such
as primary gonadal valvular insufficiency, hormonally mediated vasomotor dysfunction, and venous outflow obstruction. Central venous outflow obstruction can occur with a retroaortic left renal vein or compression of the left renal vein by the superior mesenteric artery (Nutcracker syndrome). Compression of the left common iliac vein by the right iliac artery (May–Thurner syndrome) can also cause symptomatic pelvic collaterals off of the left internal iliac vein with similar clinical presentation. Multiple approaches to the management of symptomatic PVC have been described, including hormonal suppression, hysterectomy, with or without oophorectomy, and transcatheter embolization. In appropriate patients, transcatheter embolization is now considered the gold standard for treatment of PVC.
as primary gonadal valvular insufficiency, hormonally mediated vasomotor dysfunction, and venous outflow obstruction. Central venous outflow obstruction can occur with a retroaortic left renal vein or compression of the left renal vein by the superior mesenteric artery (Nutcracker syndrome). Compression of the left common iliac vein by the right iliac artery (May–Thurner syndrome) can also cause symptomatic pelvic collaterals off of the left internal iliac vein with similar clinical presentation. Multiple approaches to the management of symptomatic PVC have been described, including hormonal suppression, hysterectomy, with or without oophorectomy, and transcatheter embolization. In appropriate patients, transcatheter embolization is now considered the gold standard for treatment of PVC.
The list of differential diagnoses for chronic pelvic pain is extensive and it is imperative that other pelvic pathology be excluded as part of the work-up for PVC. Although laparoscopy, US, computed tomography (CT), and magnetic resonance imaging (MRI) can demonstrate pelvic varices, the primary goal and utility of laparoscopy and noninvasive imaging in the work-up of PVC is to exclude concurrent nonvascular pelvic pathology. These modalities are limited by low sensitivity for the detection of primary gonadal and PVI. There is limited published data comparing laparoscopy, US, CT, and MRI to the generally accepted gold standard of catheter-directed venography. In a longer-term, 2-year study of 131 patients with a confirmed diagnosis of PCS, the sensitivities of laparoscopy, MRI, US, and CT were found to be 40%, 58.6%, 20%, and 12.5%, respectively. The investigators found no difference in clinical outcomes from embolization for PVC between those patients who had positive pre-embolization cross-sectional imaging or those patients who had directly visualized pelvic varices by laparoscopy compared to those with negative laparoscopy and negative noninvasive imaging. The authors attributed this observation to relatively low sensitivities for laparoscopy, MRI, US, and CT. It is estimated that over 80% of pelvic varices go undetected by laparoscopy due to technical limitations which include compression of the varices from peritoneal CO2 insufflation and the resultant decompression of varices while the patient is in Trendelenburg position.
Pelvic Ultrasound and Duplex
Pelvic US with duplex evaluation is an appropriate examination for screening patients with suspected PVC. The primary goal is to clearly visualize the uterus, adnexa, and ovaries. Full anatomic evaluation may require transabdominal and transvaginal techniques. Duplex evaluation should ideally be performed with the patient standing and/ or performing a Valsalva maneuver as tolerated. Venous ectasia (4 mm or greater) of the ovarian and arcuate veins of the myometrium, with slow flow (less than 3 cm/s) in the arcuate veins, and reversed ovarian vein flow are diagnostic of pathologic pelvic varicosities. It is important to note, however, that an examination negative for pelvic varicosities does not rule out PVC. In patients with a confirmed diagnosis of symptomatic PVC, pelvic varices may be demonstrated in fewer than 60% of cases by transabdominal and transvaginal US.
Magnetic Resonance Imaging and Venography
Multiplanar pelvic MRI may allow for exclusion of uterine and ovarian pathology such as endometriosis, adenomyosis, uterine fibroids, and other mass lesions. Anatomic information obtained from magnetic resonance venography (MRV) has also been described as useful in assisting preprocedure planning prior to embolization. In patients with PVC, MRV may variably demonstrate varicosities near the uterus, ovary, broad ligament, and pelvic sidewall. Gadolinium will enhance imaging and may increase sensitivity of varicosity visualization. In patients with a confirmed diagnosis of symptomatic PVC, pelvic
varices may be demonstrated in fewer than 60% of cases by MRI and MRV. The relatively low sensitivities of MRI and MRV may be partially due to technical limitations such as decompression of varices while the patient is in supine position and motion artifact.
varices may be demonstrated in fewer than 60% of cases by MRI and MRV. The relatively low sensitivities of MRI and MRV may be partially due to technical limitations such as decompression of varices while the patient is in supine position and motion artifact.
Computed Tomography and Venography
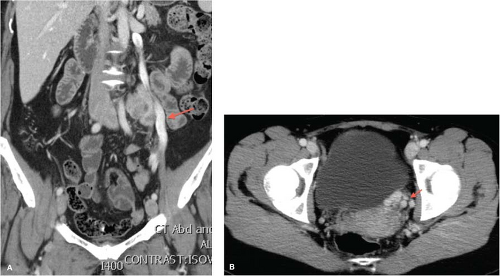
Pelvic CT with CT venography (CTV) with multiplanar reconstruction may also provide anatomic information for preprocedure planning prior to embolization and confirmation of pelvic varicosities (Fig. 13.1). CT carries a small risk of radiation exposure and necessitates the use of nephrotoxic iodinated contrast media to adequately visualize varicosities. Venous phase imaging should be obtained. In patients with a confirmed diagnosis of symptomatic PVC, pelvic varices may be demonstrated in fewer than 60% of cases by CT. Like MRI, CT is performed with the patient in a supine position, which underestimates pelvic venous distension, thus accounting for relatively low-reported sensitivities. This may be able to be partially negated if the patient is able to perform a Valsalva maneuver during image acquisition.
Endovascular Evaluation and Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree