Endovascular Treatment for May–Thurner Syndrome
Philip P. Goodney
Courtney J. Warner
Introduction
May–Thurner syndrome is defined as left leg venous outflow obstruction caused by the right common iliac artery compressing the left common iliac vein (Fig. 12.1). This phenomenon was originally described by May and Thurner in 1957 after observing asymmetry in the incidence of iliac venous thrombosis and examining 430 cadavers. May–Thurner syndrome is a primary, anatomic etiology of venous hypertension that may manifest as chronic venous insufficiency and is a risk factor for ipsilateral acute deep vein thrombosis (DVT). External compression by the iliac artery can induce vein wall hypertrophy with intimal fibrosis, spurs, and webs, potentially leading to outflow obstruction. This anatomical condition causing left leg venous hypertension is characteristic of May–Thurner syndrome, and may or may not be associated with iliofemoral DVT. Despite the symptomatic nature of patients with May–Thurner syndrome presenting to vascular surgeons, some degree of iliac vein compression may be normal. Recent studies using modern imaging techniques have documented varying amounts of iliac vein compression in 66% to 88% of the asymptomatic general public.
The classic presentation of May–Thurner syndrome is a young to middle-aged female with acute left lower extremity pain and edema secondary to deep venous thrombosis. Less commonly, patients may present with symptoms of chronic venous insufficiency, including long-standing edema, lipodermatosclerosis, and skin changes over the medial malleolus, or bursting calf pain with activity characteristic of venous claudication. External compression of the left iliac vein is an important consideration in any patient presenting with left leg symptoms, whether acute or chronic.
Chronic venous hypertension with or without postthrombotic syndrome represents a significant cause of chronic morbidity among patients with May–Thurner syndrome. Sequelae of pain, edema, hyperpigmentation, and ulceration can lead to chronic pain
and disability. Accordingly, treatment paradigms aimed at correcting venous hypertension and preventing these complications have emerged. Due to suboptimal long-term results with anticoagulation alone (approximately 20% of thrombosed iliofemoral segments will recanalize completely), endovascular techniques are used to decrease thrombus burden and correct underlying iliac vein stenosis. Catheter-directed thrombolysis (CDT), mechanical thrombectomy, and selective stent placement in this context may alleviate symptoms and decrease the risk of long-term postthrombotic morbidity. The latest American College of Chest Physicians (ACCP) guidelines advocate endovascular strategies for thrombus removal in patients with symptomatic May–Thurner syndrome and iliofemoral DVT to reduce the risk of postthrombotic syndrome.
and disability. Accordingly, treatment paradigms aimed at correcting venous hypertension and preventing these complications have emerged. Due to suboptimal long-term results with anticoagulation alone (approximately 20% of thrombosed iliofemoral segments will recanalize completely), endovascular techniques are used to decrease thrombus burden and correct underlying iliac vein stenosis. Catheter-directed thrombolysis (CDT), mechanical thrombectomy, and selective stent placement in this context may alleviate symptoms and decrease the risk of long-term postthrombotic morbidity. The latest American College of Chest Physicians (ACCP) guidelines advocate endovascular strategies for thrombus removal in patients with symptomatic May–Thurner syndrome and iliofemoral DVT to reduce the risk of postthrombotic syndrome.
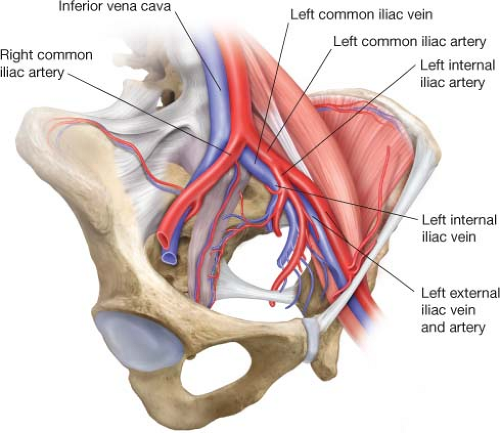 Figure 12.1 Anatomy of left iliac venous system, demonstrating the right common iliac artery crossing over the left common iliac vein. |
While catheter-directed lytic therapy can facilitate reduction of thrombus burden in the iliofemoral venous system, important contraindications exist (recent surgery or stroke, history of intracranial bleeding, etc.). Targeted drug infusion can be performed using specialized isolated pharmacomechanical thrombolysis catheters to infuse lytic agents between two occlusive balloons, thus avoiding systemic thrombolysis and hypothetically decreasing the risk of bleeding complications. Please refer to Chapter 10 for information regarding lytic techniques to treat iliofemoral DVT.
Duplex ultrasound is the primary imaging modality used to identify abnormal venous flow patterns in the femoral vein as well as diagnose DVT and quantify thrombus burden in the lower extremity. The abdominal and iliac veins may be difficult to assess due to their depth and the presence of overlying bowel gas; fasting for 4 to 6 hours prior to ultrasound may mitigate this. Abnormal venous duplex findings associated with proximal venous stenosis include continuous flow with loss of normal respirophasicity and partial or total loss of color flow in the iliac system indicating thrombosis. Augmentation is performed by compressing thigh muscles and observing appropriate changes in proximal waveforms. Comparison of duplex waveforms between the right and left iliofemoral systems is also useful to help define the laterality and extent of proximal obstruction.
Beyond identifying iliac vein stenosis, duplex ultrasound provides information on the presence and extent of DVT. If present, the proximal and distal margins of thrombus determine the appropriate puncture site and need for contralateral venous access. DVT
extending into the femoral vein requires access via the femoral vein of the thigh or popliteal vein. CT and conventional venography can provide additional anatomical information; however, both modalities require contrast administration and careful execution to obtain useful information. Formal venography is more invasive, but has the added benefit of facilitating therapeutic intervention in addition to providing diagnostic information as outlined below.
extending into the femoral vein requires access via the femoral vein of the thigh or popliteal vein. CT and conventional venography can provide additional anatomical information; however, both modalities require contrast administration and careful execution to obtain useful information. Formal venography is more invasive, but has the added benefit of facilitating therapeutic intervention in addition to providing diagnostic information as outlined below.
In addition to preoperative imaging with duplex ultrasound, a thorough history should be obtained. If the DVT was unprovoked, blood for a hypercoagulability panel should be drawn prior to anticoagulation if possible. Further, assessment of risk factors and delineation of the etiology is important to guide postprocedural anticoagulation agent choice and duration.
Endovascular intervention for May–Thurner syndrome is ideally performed in an angiography suite (Fig. 12.2) with nursing staff available for medication administration and monitoring of vital signs, a radiology technician to assist with imaging, and a surgical assistant to facilitate management of wires/catheters (the last two roles may be combined). Standard backtable set-up includes a micropuncture kit, a 0.035-in J-wire with a 5-Fr flush catheter for venography, as well as heparinized saline flush and contrast syringes (Fig. 12.3




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


