Chapter 72
Endovascular Therapy of Vascular Malformations
Carlos J. Guevara, Ahmad Alomari
Management of vascular malformations has evolved over time to encompass three distinct approaches: the conservative, the minimally invasive, and the operative. Percutaneous interventional management has become widely accepted as the principal minimally invasive therapy for vascular malformations, replacing surgery for many traditional indications. Nevertheless, many challenging vascular malformations necessitate therapeutic strategies that combine some or all of these approaches. Management of vascular anomalies can be challenging and lengthy; these disorders are extremely heterogenous, affect any body organ or system at any age, and have frequently overlapping presentations. Although teamwork is not a substitute for individual innovation, these disorders are best managed with an interdisciplinary approach managed by relevant medical specialists at specialized centers.1
Essential steps in the treatment algorithm for vascular malformations include accurate diagnosis and characterization of the particular malformation and proper expectations for the treatment approach.
In this chapter we review the different percutaneous treatment options for both the low-flow (venous and lymphatic) and the high-flow (arteriovenous) vascular malformations.
Venous Malformations
Venous malformations (VMs), the most common vascular malformations, manifest typically as solitary lesions with preference for the cervicofacial area, extremities, and trunk.2,3 Multiple lesions may have a genetic predisposition (such as TIE2 mutation in cutaneomucosal venous malformation and glomulin mutation in glomuvenous malformation).4 Blue rubber bleb nevus syndrome is a sporadic multifocal venous malformation involving the soft tissue and gastrointestinal tract.
It is of paramount importance to differentiate between two main morphologic types of VMs: the spongiform mass type and congenital ectasia of veins (phlebectasia). The latter carries a significantly higher risk of thromboembolism, which can be largely prevented with early therapeutic measures. Phlebectasia can be sporadic but is commonly encountered in complex overgrowth syndromes (e.g., Klippel-Trenaunay and CLOVES [congenital, lipomatous, overgrowth, vascular malformations, epidermal nevi and spinal/skeletal anomalies and/or scoliosis] syndromes) or other extremity anomalies (e.g., fibroadipose vascular anomaly [FAVA]).
Pain in VMs is multifactorial, with acute episodes principally predisposed by clot formation. Symptoms may worsen with menarche and pregnancy. Swelling and engagement of venous spaces and involvement of joints, muscles, and tendons are also contributing factors. Particular anatomic locations bear additional specific morbidities. For example, articular synovial involvement, particularly around the knee joint, predisposes to hemarthrosis and premature degenerative chondropathy. Airway lesions can cause obstruction, and gastrointestinal lesions can manifest as bleeding. Extensive osseous and chondral involvement may eventually lead to bone deformity, pathologic fracture, and joint failure.
Sclerotherapy for Venous Malformations
Indications
The prime indication for treatment of VMs is pain, which eventually develops in the vast majority of lesions. Lesions in particular locations (e.g., synovial-articular), symptomatic gastrointestinal VMs, and disfiguring (e.g., facial, genital) VMs are treated even in the absence of pain. Treatment of such lesions should be initiated early in life, before they enlarge, because smaller lesions may require fewer procedures and smaller volumes of sclerosants and because early treatment may prevent some long-term complications. Painless small, nondisfiguring VMs can be managed conservatively, as can painless very diffuse or extensive VMs.
Preoperative Evaluation
Localized intravascular coagulation (LIC) is common with large VMs, causing elevation of D-dimer, hypofibrinogenemia, and, to a lesser extent, borderline low or slightly decreased platelet count.5 Basic coagulation parameters (complete blood count, prothrombin time/international normalized ratio, partial thromboplastin time, fibrinogen and D-dimer measurements) are usually obtained prior to interventions for large VMs. Correcting minor hematologic abnormalities is not necessary before percutaneous interventions.
Characterization of the size and extension of a lesion can be performed with ultrasonography (US) and magnetic resonance imaging (MRI). Ultrasonographically, VMs appear as hypoechoic or anechoic compressible spaces containing stagnant blood within thin anomalus venous walls. Other than the hyperechoic clots or phleboliths, VMs have no substantial solid components (Fig. 72-1A). Blood flow in spongiform VMs is not usually seen on color-flow Doppler ultrasonography. MRI sequences should be obtained with fat saturation and should include T1-weighted, T2-weighted, and post-contrast T1-weighted sequences. As on US, VMs are depicted on MRI as blood-containing spaces with phleboliths (Fig. 72-1B). Fluid-fluid levels are very common. Post-contrast enhancement is patchy and heterogenous. MR angiography (MRA) and MR venography (MRV) are not usually helpful for spongiform VMs. Intralesional or ascending venography and CT scans are of limited diagnostic use (Figs. 72-1C, 72-2).
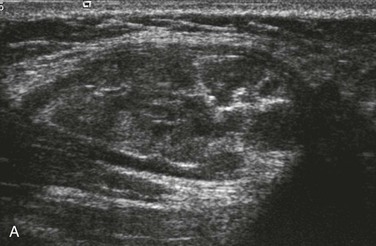
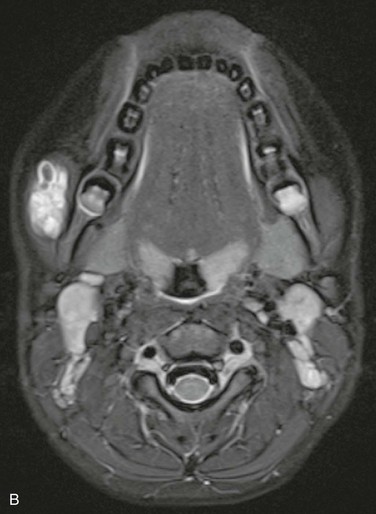
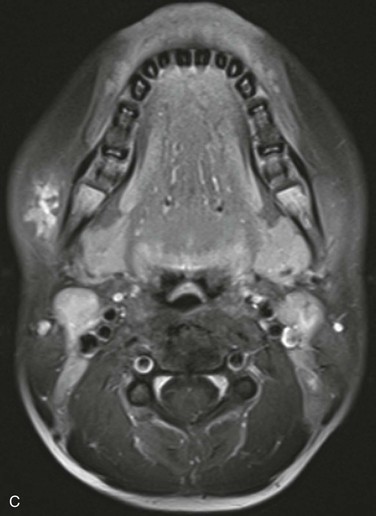
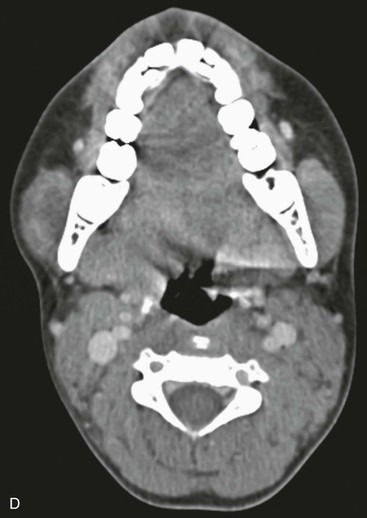
Figure 72-1 Venous malformation of the masseter muscle. A, Ultrasonography of the right cheek shows an intramuscular, hypoechoic, fluid-filled space containing internal septations and multiple phleboliths. B, Axial fat-saturated T2-weighted MR image. The VM is well defined and hyperintense and contains hypointense oval phleboliths. C, Post-contrast axial fat-saturated T1-weighted MR image demonstrating partial, heterogenous enhancement. D, Post-contrast CT scan demonstrated the hypodense VM in the left masseter muscle. The details of soft tissue are inferior to those shown by the MR images.
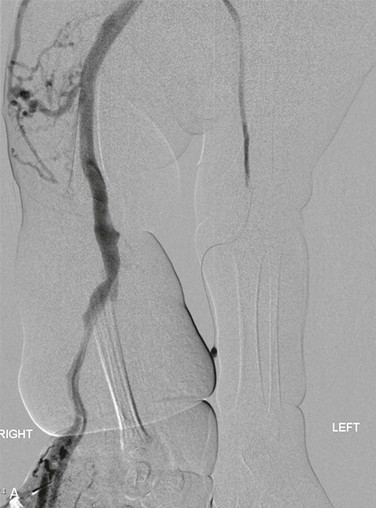
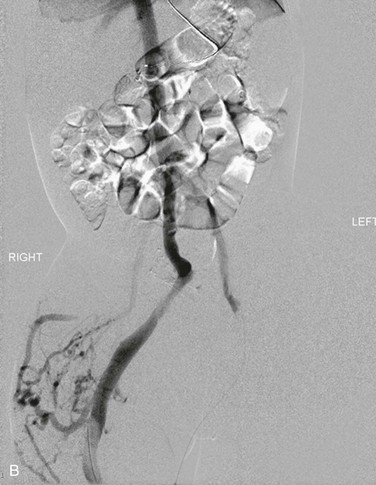
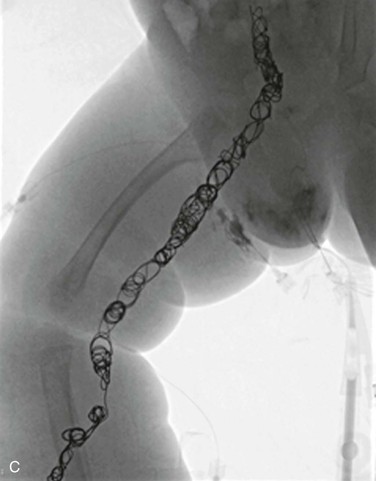
Figure 72-2 A, Venography of the lower extremities in a child with CLOVES syndrome. Note the markedly ectatic marginal and sciatic veins in the right leg. The left femoral vein is normal. Malformation of the right flank. B, Venography of the pelvis and abdomen. The sciatic and internal iliac veins are ectatic. Common femoral and external iliac veins are normal bilaterally. C, Coil embolization of the ectatic marginal, sciatic, and internal iliac veins.
Sclerosing Agents
The widely used agents for sclerotherapy of VMs include dehydrated ethanol, sodium tetradecyl sulfate (Sotradecol, AngioDynamics, Queensbury, NY), polidocanol (Asclera, Chemische Fabrik Kreussler & Co. GmbH, Wiesbaden, Germany), sodium morrhuate and ethanolamine oleate (Ethamolin, QOL Medical, Vero Beach, Fla) and alcohol solution of zein (Ethibloc, Ethnor Laboratories/Ethicon, Norderstedt, Germany).
Permanent embolic agents, such as N-butyl cyanoacrylate glue and coils, are used for percutaneous closure of phlebectasia, capacious venous spaces or draining veins (Fig. 72-3). Preoperative glue embolization of VM may also be used to facilitate the surgical resection in selected cases. Endovenous laser treatment can also be used in combination with sclerotherapy.
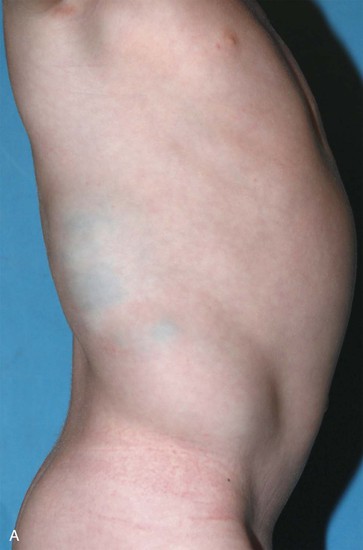
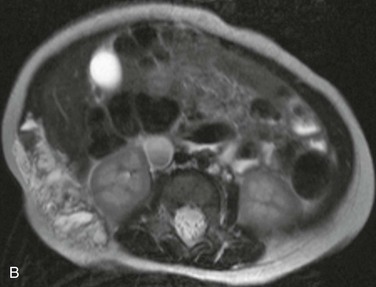
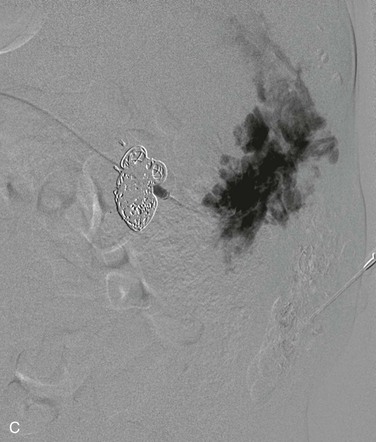
Figure 72-3 Venous malformation of the right flank. A, Photograph of the lesion. B, Axial T2-weighted MR image depicting the full-thickness involvement of the abdominal wall muscles. C, Intralesional venography. Amorphous stagnant blood-filled spaces can be seen draining via lumbar and intercostal veins. Coils were used to obliterate a large inferior vena cava aneurysm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


