CHAPTER 72 Endovascular Therapy for Thoracic Aortic Aneurysms and Dissections
The surgical management of thoracic aortic aneurysms began in the 1950s with the introduction of cardiopulmonary bypass technology.1–4 Since then, advances in surgical technique and perioperative management have resulted in improved outcome in the management of thoracic aortic disease. Despite these advancements, the morbidity and mortality associated with open thoracic aortic repair remain significant. Furthermore, our population of patients has become increasingly older with more significant comorbidities. This has led to the development of thoracic endovascular aortic repair (TEVAR) in an effort to improve perioperative outcome and potential long-term survival.
Endovascular treatment of aortic disease was introduced in the early 1990s and has dramatically revolutionized the field of cardiovascular surgery. Since Parodi’s first description of an intraluminal stent graft device for the treatment of abdominal aortic aneurysms,5 stent graft device technology has been expanded to treat the multiple pathologic processes in the thoracic aorta. In 1994, Dake first reported the initial Stanford experience with 13 patients undergoing endovascular therapy for descending thoracic aortic aneurysms.6 Since then, indications involving off-label use have expanded to include the treatment of aortic dissections, traumatic transections, penetrating atherosclerotic ulcers, and intramural hematoma. Currently, there are three stent graft devices approved by the Food and Drug Administration (FDA) for the treatment of descending thoracic aortic aneurysms. However, concerns and questions remain about the appropriate timing, indications for intervention, and durability of this fast-evolving technology. Furthermore, morbidity and mortality compared with the standard open repair remain unclear. This chapter reviews this fast-growing field that has gained worldwide acceptance.
THORACIC AORTIC ANEURYSMS
Natural History and Indications for Intervention
Thoracic aortic aneurysms are abnormal dilations of the aorta characterized by elastin fragmentation and fibrosis, resulting in medial degeneration of the aortic wall.7 These age-related changes are likely to result in the reduction of aortic integrity and strength. As our population is ever increasing in age, the incidence of thoracic aortic aneurysms also appears to be increasing. In a Swedish study examining the national health care registry from 1987 to 2002, the incidence of thoracic aortic disease rose by 52% in men and by 28% in women, reaching 16.3 per 100,000 per year and 9.1 per 100,000 per year, respectively. In the same study, the annual incidence of operations performed on the thoracic aorta in men increased from 0.8 per 100,000 per year in 1987 to 5.6 per 100,000 per year in 2002 for an overall 7-fold increase; in women, the increase was 15-fold, from 0.2 per 100,000 per year in 1987 to 3.0 per 100,000 per year in 2002.8
The natural history of thoracoabdominal aortic aneurysms has been examined in large single-institution series. Characterized by slow growth over time, aortic aneurysmal degeneration is an indolent pathologic process of aortic dilation leading to potential rupture or dissection. The human aorta grows generally at a rate of about 0.07 cm per year in the ascending aorta and 0.19 cm per year in the descending thoracoabdominal aorta.9 If dissection is present, the thoracoabdominal aorta may grow at a slightly faster rate of 0.28 cm per year.10,11
The major risk in thoracic aortic aneurysms pertains to the catastrophic events of rupture and dissection. The risk of rupture or dissection as a function of maximum aortic diameter has been examined in multiple studies. Clouse and coworkers12 examined the Olmstead County database and demonstrated that in patients with a maximum aortic diameter between 4.0 and 5.9 cm, the risk of rupture was 16% during a period of 5 years. With a maximum aortic diameter greater than 6.0 cm, the risk of rupture exceeded 30% during the same period of 5 years. The Yale group has also identified “hinge points” of maximum aortic diameter that represent significant increases in the risk for rupture. In the ascending aorta, this hinge point appears to be at 6 cm, with a risk of rupture or dissection of 34% during the lifetime of the patients. In the descending thoracic aorta, the hinge point appears to be at 7 cm, with a 43% risk of rupture or dissection.10 The annual risk of rupture or dissection as a function of maximum aortic diameter has also been examined. Davies and coauthors9 demonstrated that in patients with a maximum aortic diameter of less than 6.0 cm, the yearly rate of rupture, dissection, or death is less than 8%. However, the annual risk of rupture, dissection, or death dramatically increases to 15.6% when the maximum aortic diameter is 6.0 cm or greater.
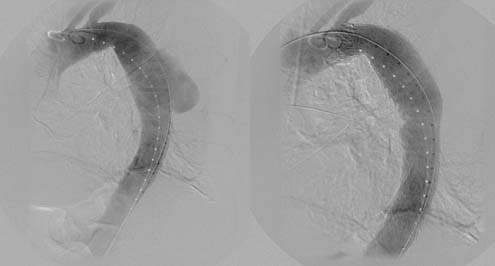
In the era of TEVAR, the perceived lower rates of morbidity and mortality have urged the question, Should the threshold for intervention in the descending thoracic aorta be lowered? With a mortality of less than 5% with TEVAR in most centers of excellence,13–16 most patients with a descending thoracic aortic diameter greater than 5.5 cm may be considered for endovascular repair (Fig. 72-1). However, the final decision to intervene must be based on the previously established surgical dictum: the benefit of surgery must outweigh the risk of rupture, regardless of approach.
Device Development
Since the early devices were first described by the Stanford group in 1994,6 TEVAR devices have undergone multiple modifications and clinical trials. Three thoracic endoprostheses are currently approved for the treatment of descending thoracic aortic aneurysms. Second- and third-generation devices as well as disease-specific devices are currently being investigated.
Gore TAG
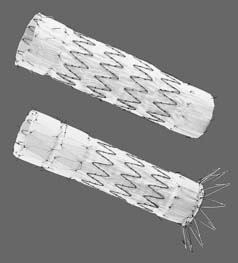
The Gore TAG thoracic endoprosthesis (W. L. Gore & Associates, Flagstaff, AZ) was approved by the FDA in March 2005 for the treatment of descending thoracic aortic aneurysms. The first device to be approved, the Gore TAG is a self-expanding expanded polytetrafluoroethylene endograft composed of nitinol support. Concern about fracture of the longitudinal support wire led to the revision of the original Gore device to its current Gore TAG endoprosthesis. Unique to this device system is the introducer sheath, which enables the delivery of multiple devices and minimizes traumatic injury to the femoral access vessels. The introducer sheath ranges from an inner diameter of 20 Fr to 24 Fr depending on the size of the Gore TAG devices required for treatment. The Gore TAG endoprosthesis ranges from a minimal diameter of 26 mm to a maximum diameter of 40 mm, with lengths ranging from a minimum of 10 cm to a maximum of 20 cm (Fig. 72-2). The mechanism of deployment involves release of the endograft from a center to peripheral fashion. Thus, precise deployment of the proximal and distal landing zones may be challenging.
Medtronic Talent and Valiant Thoracic Endoprostheses
The Talent Device
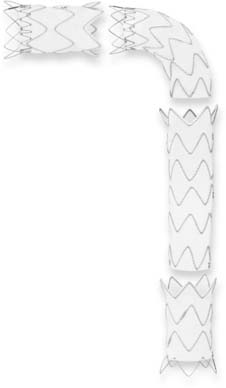
The Talent device is designed as a modular system; 47 different configurations ranging from a diameter of 22 to 46 mm and cover lengths from 112 to 116 mm are available. To accommodate the size differences often found between the proximal and distal portions of the aorta in thoracic aneurysms, tapered grafts are available for better aneurysmal conformability and prevention of junctional endoleaks. Four configuration categories are available: proximal main, proximal extension, distal main, and distal extension (Fig. 72-3). The proximal configurations and the distal extension are offered with a bare spring design (Free-Flo design). The bare spring design allows placement of the device crossing the arch vessels proximally and the celiac artery distally for suprasubclavian and infraceliac fixation, respectively.
Cook Zenith TX2 Thoracic Endoprosthesis
Approved in 2008 by the FDA, the Cook Zenith TX2 thoracic endoprosthesis (Cook Inc., Bloomington, IN) is designed as a modular system with a specific proximal and distal configuration (Fig. 72-4). The grafts consist of stainless steel Z-stents with full-thickness polyester fabric. Similar to the Medtronic delivery system, the Zenith TX2 system does not require a delivery sheath, and it is introduced as a preloaded catheter with triggers. The device sheath has a hydrophilic coating, and sizes range from 18 Fr to 22 Fr, depending on the diameter of the endoprosthesis. The diameter of the endoprosthesis ranges from 22 to 42 mm, and lengths range from 120 mm to 207 mm. The two components are designed to be deployed from a proximal to distal direction, and tapered devices are available.
Anatomic and Technical Considerations
Access
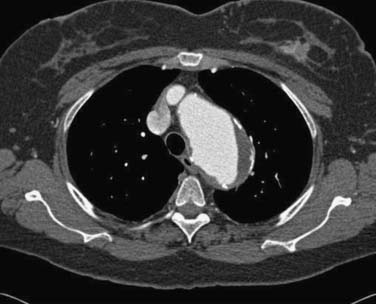
Safe vascular access for thoracic aortic device deployment is the key to thoracic aortic stent grafting. The majority of the morbidity and mortality is a direct result of arterial access complications.13,14,16 Extensive preoperative planning with appropriate imaging is mandatory. The “gold standard” for preoperative evaluation is computed tomographic (CT) angiography, which includes the thorax, abdomen, pelvis, and femoral arteries. Fine-cut helical CT scanning with a minimum of 3-mm slices is ideal (Fig. 72-5). In those patients who cannot undergo CT scanning with the administration of contrast material, magnetic resonance angiography is an acceptable substitution.
All of the thoracic endovascular devices are long to reach the descending aorta, of large caliber to contain the thoracic aortic endoluminal graft, and relatively stiff to allow “pushability” through the iliofemoral access points and through the abdominal aorta. The management of the delivery of the thoracic aortic stent graft is often the most challenging aspect of the case. Delivery systems of the three currently FDA-approved thoracic endoprostheses will require arterial access size of a minimum of 7.5 to 8.0 mm. Creation of a conduit to the femoral or iliac artery may be necessary to achieve adequate access. Not only the size of the arterial vessels but also the anatomy of the iliofemoral and abdominal aorta must be considered in planning the access route. Excessive tortuosity and atherosclerosis with occlusive disease may provide barriers to safe delivery of the endograft. In approximately 20% of patients, a retroperitoneal access to the common iliac arteries will be required because of issues of femoral or external iliac artery size and tortuosity.17
The removal of the large sheath, especially when it is inserted with some force and manipulation, is a particularly dangerous period when injury may occur. There have been numerous reports of successful thoracic endografting with a sheath removed with a complete iliac artery avulsed and attached (Fig. 72-6). At the time of recognition, a stiff wire through this injured artery may be lifesaving and allow insertion of an occluding balloon proximally for control of a potentially life-threatening bleed. In addition, both the blood pressure and heart rate should be carefully monitored during removal and completion of the endovascular procedure for signs of an occult injury.
Landing Zones
The proximal aorta is divided into landing zones as illustrated in Figure 72-7. Unless revascularization is performed, proximal landing in zone 0 and zone 1 is unacceptable because of the necessary occlusion of the left common carotid artery in zone 1 and the innominate artery in zone 0. Proximal landing in zone 2 is commonly used with either partial or total occlusion of the left subclavian artery. Zone 3 landing is dependent on the exact anatomic neck at the arch. Proximal landing in zone 3 can lead to angulation of the graft, which provides inadequate sealing of the proximal graft along the lesser arch and “bird beaking” or “stove piping” graft placement, resulting in a high incidence of type II endoleaks. Zone 4 landing is usually straightforward because of the lack of angulation and distance from the arch vessels.
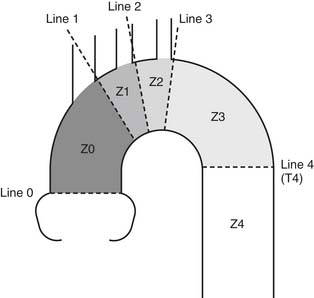
Figure 72–7 Classification of landing zones in thoracic aortic endovascular repair.
(From Mitchell RS. First International Summit on Thoracic Aortic Endografting. J Endovasc Ther 2002;9:II98-105.)
Because zone 2 is often the site of best proximal landing to avoid excessive angulation and tortuosity, the management of the left subclavian artery requires preoperative planning. Some of the possible complications of covering the left subclavian artery include vertebrobasilar artery insufficiency or stroke, left arm ischemia, and ischemia of the heart in patients with a previous left internal mammary artery to left anterior descending coronary artery bypass graft. An evaluation of the right vertebral artery and the adequacy of the circle of Willis is crucial in planning to cover the left subclavian artery. In those patients with inadequate collaterals through the circle of Willis, stenotic right vertebral artery, or dominant left vertebral artery, strong consideration should be given to bypass of the left subclavian artery before covering. One option is to transpose the left subclavian artery to the left common carotid artery with oversewing of the proximal left subclavian artery. A second option is to bypass from the left common carotid artery to the left subclavian artery. Either surgical ligation of the proximal left subclavian at the time of bypass or staged coil embolization of the proximal left subclavian artery at the time of graft delivery may be used. Bypass of the left subclavian may be preferable because it avoids any mediastinal dissection, and there is no interruption of antegrade flow to the vertebral artery or internal mammary artery branch vessels.18
Results
Multicenter Clinical Trials
The Gore TAG endoprosthesis was approved as a result of the phase II U.S. multicenter trial comparing the Gore TAG endoprosthesis with an open surgical control group in the treatment of descending thoracic aortic aneurysms.13 Between September 1999 and May 2001, 140 patients with descending thoracic aortic aneurysms were enrolled from 17 sites in the United States. All patients were required to have adequate landing zones of at least 2 cm in length of nonaneurysmal aorta distal to the left carotid and proximal to the celiac axis. At the same centers, the open surgical control cohort enrolled 94 patients; 44 patients were concurrent controls and 50 were historically and retrospectively acquired by selection of the most recent surgical patients in reverse chronologic order.
In the endograft group, statistically significant improvement was seen in the 30-day mortality, incidence of postoperative respiratory failure, renal failure, spinal cord ischemia, mean intensive care unit stay, and hospital stay. Thirty-day mortality was 2.1% and 11.7% in the Gore TAG group and the open surgical control group, respectively. Spinal cord ischemia was 2.9% in the Gore TAG group versus 13.8% in the control group. However, peripheral vascular complications were significantly higher in the endograft group (14% versus 4% in the control group).13
The mean duration of follow-up was 25.8 months in the endograft group and 24.9 months in the open control group. Kaplan-Meier 2-year survival was similar between the two groups (78% in the endograft group versus 76% in the control group). The incidence of endoleak at 1-year and 2-year follow-up was 6% (6 of 103) and 9% (7 of 80), respectively. There were no cases of aneurysm rupture in either group. At 2-year follow-up, 45% of the endograft group had aneurysmal regression of 5 mm or more, 42% had no change, and 13% had aneurysmal progression of 5 mm or more.13 At 5-year follow-up, all-cause mortality remained similar between the two groups (68%, endograft group; 67%, control). Aneurysm-related mortality was lower in the endograft group (2.8%) compared with the control group (11.7%).19
Published in 2008, the international controlled clinical trial of the TX2 device is a nonrandomized, controlled, multicenter, international trial comparing the treatment of thoracic aortic aneurysm with the TX2 endoprosthesis versus open repair. Enrollment began in March 2004 and was completed by July 2006. Involving 42 institutions, a total of 160 patients were enrolled in the endovascular group and 70 patients in the open group. The 30-day survival rate was noninferior for the TX2 group compared with the control group (98.1% versus 94.3%). The 30-day cumulative major morbidity scores were significantly lower in the TX2 group than in the control group (1.3 versus 2.9). Although not statistically significant, the incidence of neurologic complications trended in favor of endovascular repair. The incidence of stroke in the TX2 group was 2.5% at 30 days compared with 8.6% in the control group. The incidence of paraplegia in the TX2 group was 1.3% versus 5.7% in the control group. At 12 months of follow-up, aneurysmal growth was seen in 7.1%, endoleak in 3.9%, and device migration in 2.8%. One-year survival estimated from all-cause mortality was similar between the TX2 group (91.6%) and the control group (85.5%). The 1-year survival estimate from aneurysm-related mortality was also similar between the TX2 group (94.2%) and the open surgical control group (88.2%).16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree