Chapter 35 Endovascular Repair of Thoracic Aortic Aneurysm
Thoracic aortic aneurysms (TAAs) are less frequent than abdominal aneurysms but are no less significant. The estimated incidence is 10.4 cases per 100,000 population.1 With the aging population in the United States coupled with advances in imaging technology, there has been a steady increase in the number of diagnosed TAAs. The natural history of these aneurysms is not well characterized, although it has been shown that approximately 70% of patients who forgo treatment will progress to rupture, with a fatality rate approaching 90%.2
Traditionally, repair of TAAs has been limited to open aortic replacement with significant morbidity related to thoracotomy, single lung ventilation, aortic cross clamping, and prolonged visceral or renal ischemia with a prolonged hospital stay and recovery. The reported mortality rates with open repair of TAA at centers of excellence have ranged from 3% to 8%, with paraplegia rates of 3% to 5%.3,4 The first endovascular exclusion of a thoracic aorta was reported by Dake in 1994.5 Development of commercial thoracic endografts, however, was slow because of the relatively low prevalence of thoracic aneurysms, the hostile hemodynamic forces of the thoracic aorta and the need for large devices and delivery systems. The U.S. Food and Drug Administration (FDA) approved the first thoracic endograft in March 2005 after a nonrandomized trial showed that thoracic endovascular repair (TEVAR) compared favorably to the standard open procedure. Since then, there has been a paradigm shift in the treatment of descending thoracic aneurysm (DTA), with high-risk patients being offered TEVAR, thus expanding the pool of patients that could undergo treatment. Despite the lack of randomized trial data comparing it with open surgical repair, TEVAR has become the preferred method of treatment for all DTA.
Indications for Thoracic Endovascular Repair
The decision to intervene on a DTA depends on its size, location, rate of growth, and symptoms and the overall medical condition of the patient. The indications for TEVAR should not differ from those for open repair and typically include aneurysms greater than 6 cm in diameter. Saccular and symptomatic aneurysms are often repaired at a smaller size. It is also suggested that aneurysms with a rapid growth rate (more than 1 cm per year, or 0.5 cm in 6 months) should be considered for early repair.6 TEVAR has also been used in other types of thoracic pathology, including acute complicated type B dissection, traumatic thoracic aortic injury, large penetrating aortic ulcers, and intramural hematomas7 (Box 35-1). The discussion in this chapter will be limited to aneurysmal disease.
Preoperative Planning: Imaging
The decision to proceed with TEVAR requires detailed imaging for procedural planning. Important anatomic characteristics include landing zones, vessel tortuosity, aneurysm location in relationship to branch vessels, and access size and quality. Computed tomographic angiography (CTA) is the modality of choice for evaluation of aortic pathology and TEVAR planning. Multiplanar reconstructions and three-dimensional modeling provide invaluable information regarding vessel sizes, angulation, length, and anatomic relationships as well as severity of calcification and presence of thrombus. The availability of advanced software such as Vitrea (Vital Images, Minnetonka, Minn.) and Aquarius (TeraRecon, San Mateo, Calif.) has brought the power of workstation analysis to simple desktop computers, allowing operators to perform accurate graft sizing and predeployment planning. For patients who cannot undergo CTA safely, magnetic resonance angiography has also been used successfully, although patients with renal failure are at risk for nephrogenic systemic fibrosis.8
Anatomic Considerations
Landing Zones
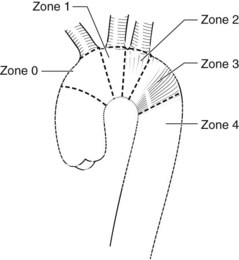
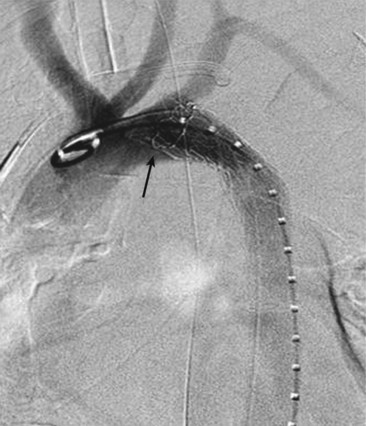
The proximal seal locations have been classified into five zones (Figure 35-1). Each zone is divided by a tangential line along the distal side of each great vessel; zone 0 involves the origin of the innominate artery, zone 1 the origin of the left common carotid artery (LCCA), zone 2 the origin of the left subclavian artery (LSA), zone 3 the proximal descending thoracic aorta down to the T4 vertebral body, and zone 4 the remainder of descending thoracic aorta. The location of the proximal landing zone depends on the aneurysm morphology; however; the ideal location in terms of anatomic accommodation of the graft is in zones 3 and 4. An important consideration for the proximal seal zone is the angle of the aortic arch. Grafts that are placed within the aortic arch (zones 2 and 3), especially one with an acute angle, may fail to properly appose to the inner curvature of the arch (Figure 35-2). This configuration has occasionally resulted in untoward effects such as migration and collapse, mostly in nonaneurysmal applications. Recent endograft modifications such as the Zenith Pro-Form (Cook, Bloomington, Ind.) and the C-TAG (W.L. Gore, Flagstaff, Ariz.) have been designed to specifically address this problem.
Management of the Left Subclavian Artery
In approximately 20% of cases, adequate coverage of the TAA can be achieved only by extending the graft into zone 2, effectively excluding the LSA from circulation9 (Figure 35-3). The management strategy of the LSA has evolved since the inception of TEVAR. Prophylactic revascularization of the LSA, either through a carotid subclavian bypass or a transposition, was used routinely in the first study of TEVAR. Intentional coverage without revascularization was later tolerated as a relatively safe practice especially in emergency settings and in the presence of good collateral circulation. Absolute contraindications include an occluded or atretic right vertebral artery or a left internal mammary bypass to the coronary circulation. A review of 22 patients who underwent intentional coverage of the LSA found that almost 70% of the patients remained asymptomatic, with the remaining 30% suffering only mild left arm claudication symptoms.
The practice of LSA coverage has come under severe scrutiny lately with EUROSTAR data linking it to an increased risk of spinal cord ischemia. Several reports of brain stem strokes and other complications have also surfaced. Peterson and colleagues11 observed a 63% (5/8) stroke and upper extremity ischemic symptom rate in patients who had LSA coverage without revascularization. A review of a random segment of the literature disclosed a 23% complication rate with LSA coverage compared with a 3% rate when flow to the LSA was maintained.11,14 The current Society for Vascular Surgery (SVS) guidelines recommend routine preoperative revascularization in elective TEVAR and expectant management in acute settings excluding absolute contraindications.15
Management of the Celiac Axis
The ideal distal landing zone places at least 2 cm of graft proximal to the celiac axis. When the aneurysm is more extensive, coverage of the celiac artery is a possibility, allowing for a longer seal zone. Concerns for bowel ischemia and hepatic failure should prompt careful assessment of the collateral network, especially through the gastroduodenal and pancreaticoduodenal arcades. A recent review reported selective coverage of the celiac axis in 31 patients during TEVAR.16 Of these patients, two (6%) patients had bowel ischemia, and five (16%) had an endoleak that required reintervention. The findings illustrate the fact that celiac coverage is feasible in many cases, but potentially morbid in the absence of adequate collaterals, and should be undertaken with caution.
Stent Graft Description
Gore TAG
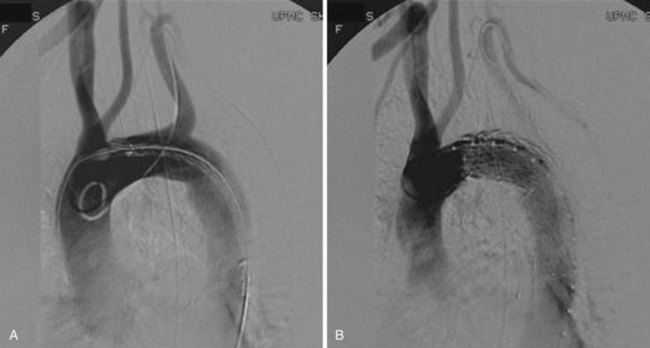
The TAG graft (W.L. Gore, Flagstaff, Ariz.) was the first FDA-approved device for the treatment of DTAs (Figure 35-4). As such, it is the most common device implanted for both on- and off-label use in the United States. The graft is an expanded polytetrafluoroethylene (ePTFE) tubular graft reinforced with a layer of fluorinated ethylene propylene (FEP) material and external Nitinol self-expanding stents. The marketed TAG endograft has a lower porosity to help avoid the sac enlargement associated with the earlier version of the device.17,18 The FEP wrap was also added as a replacement of the longitudinal deployment wire tested with the original device. The stent wire-forms are secured to the graft with a bonding tape made of ePTFE and FEP. Effective proximal and distal seals are achieved by flared edges of the graft as well as an additional ePTFE cuff. The graft has an inlaid gold band that marks the proximal and distal edges, although the flared ends protrude 7 to 9 mm beyond these edges.
The new conformable TAG (C-TAG) device has been modified to increase conformability, especially to the lesser curvature of the arch by changing the wire form strength and pattern (Figure 35-5). The proximal covered scallops have also been replaced by short bare metal stents ranging in length from 3 to 6.5 mm. A 21-mm device has been added to the size range so that patients with smaller aortic diameters can be treated. Two tapered devices (26 to 21 mm and 31 to 26 mm) were also added, providing added flexibility in sizing. Other changes include the addition of a 46-mm device in 2010 and the introduction of a new pressurized hemostatic valve on its sheath to minimize blood loss.
Medtronic Talent
The graft is composed of a nitinol skeleton attached to a woven polyester graft. There is a bare metal component at the proximal end to allow for extended fixation while maintaining flow to the branch vessels. There are a number of tapered and nontapered configurations available as well as different configurations for the last stent (Figure 35-6). The support bar of the device is intended to be aligned along the greater curvature of the aorta, and can be identified by the radiopaque “8” that marks the bar. The Talent is available in a wide range of diameters, from 22 to 46 mm, but was initially limited to 112- to 116-mm lengths, which required many devices to be used to cover a long aortic segment, an average of 2.7 devices in the VALOR trial.18,19 The device is usually oversized by 10% to 20% over the aortic landing zones and uses a 22- to 25-French delivery catheter.
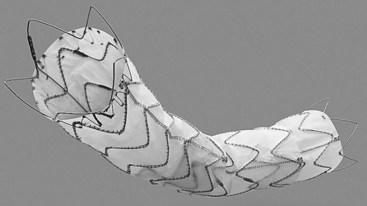
FIGURE 35-6 Medtronic Talent device.
(From Moore WS: Endovascular surgery, ed 4, Philadelphia, 2011, Saunders.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree