Chapter 40 Endovascular Repair of Juxtarenal (Chimney), Infrarenal, and Iliac Artery Aneurysms
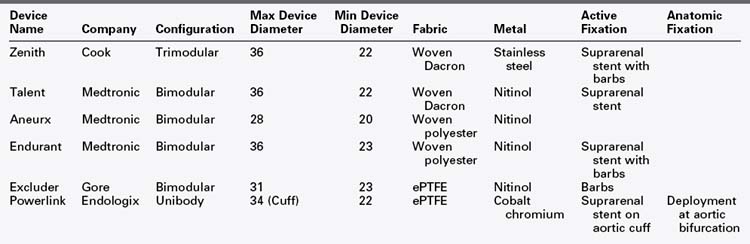
In 1991, Parodi and colleagues1 published a seminal study of patients who underwent abdominal aortic aneurysm repair using an intraluminal, stent-anchored polyester prosthetic graft delivered retrograde from the common femoral artery and revolutionized the field of vascular surgery. While initially considered the preferred modality for patients deemed unfit for open surgery, endovascular aneurysm repair (EVAR) has rapidly evolved as an important alternative, less invasive, and frequently preferred treatment for patients with abdominal aortic aneurysms. The technology has significantly reduced perioperative morbidity and mortality resulting in expanded application, and the wide acceptance by physicians has been the impetus for subsequent device evolution. Since its inception, the technology has undergone multiple iterations, with earlier generation grafts having been abandoned in favor of current endografts with their superior radial force, columnar support as well as additional modes of fixation. There are currently six endografts approved by the U.S. Food and Drug Administration (FDA) and on the market for the treatment of infrarenal abdominal aortic aneurysms (Table 40-1). The endograft consists of a metal stent attached to prosthetic graft material which is housed in a sheath, to allow for intraarterial delivery of the device. Most bifurcated stent-grafts use a modular design, the exception being the Endologix device in which the bifurcated component is deployed in a unibody fashion. The basic differences in construct are outlined in Table 40-1.
Setting
Traditionally, endovascular abdominal aortic aneurysm repair has been done in an interventional or catheterization suite. Currently, however, the hybrid operating room with fixed C-arm positioning affords greater flexibility and safety. Alternatively portable C-arm imaging systems are far more sophisticated than when many clinicians began performing EVAR in the mid 1990s. EVAR has been described with regional block, local anesthesia, and general anesthesia, but the authors believe that general anesthesia gives the surgeon maximal control over the operation with minimal risk to the patient. Having immediate access to an open vascular set is paramount and at times lifesaving should the patient need to be converted emergently. It is also useful if attendant open adjunctive procedures such as femoral-femoral artery bypass grafting, iliac conduit, or local femoral endarterectomy need to be performed. As EVAR is being performed increasingly in much older comorbid patients with less than ideal anatomy, the need for sophisticated operative imaging to optimize precision deployment, as well as a surgical preparedness for potentially catastrophic access issues, underscores the importance of the operating room or hybrid suite in performing these procedures. The outcomes of EVAR for ruptured abdominal aortic aneurysm (AAA) have also been favorable, particularly in institutions where a systematic algorithm ensures that the appropriate endovascular team is alerted when a ruptured aneurysm is identified in the emergency room.2,3 It is clear that, in this subset of patients, having the ability to convert from an endovascular to an open approach should they rapidly deteriorate is an important consideration. Of paramount importance to the success of every aortic endovascular program is identifying and training a dedicated endovascular team of nurses, operating room scrub technicians, and radiology technologists. For many institutions this will require a major shift in operating room culture.
Endovascular Stent Graft Planning and Placement for Infrarenal Aortic Aneurysms
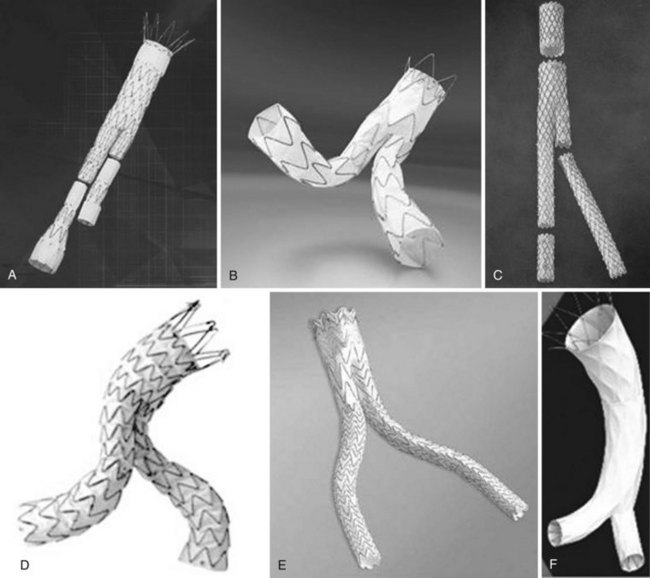
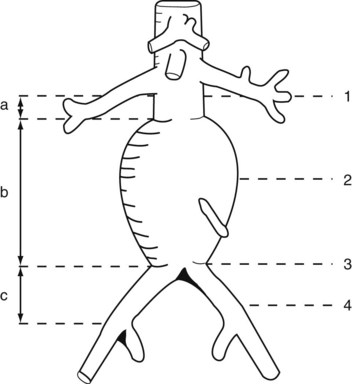
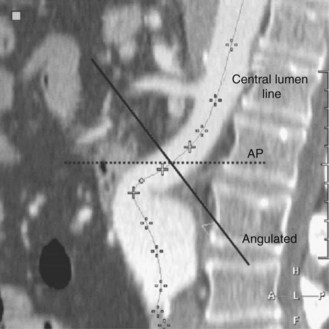
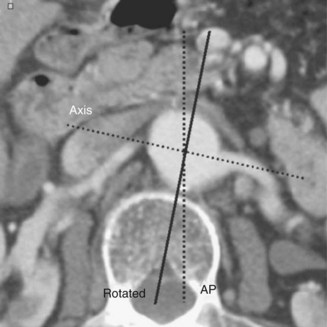
The majority of the case is performed before entering the operating room. Axial imaging is obtained via computed tomography angiography or magnetic resonance angiography provided that the patient does not have renal insufficiency. Three-dimensional reconstructions performed by either Terra Recon (Foster City, Calif.), Vitrea (Vital Images), Leonardo (Siemens), or M2S (Lebanon, N.H.) have become the standard and are used to measure the distance from the lowest renal artery to the aortic bifurcation, the lowest renal artery to the ipsilateral hypogastric artery, and the lowest renal artery to the contralateral hypogastric artery (Figures 40-1 and 40-2). Diameter measurements of the neck and of the common iliac arteries are then performed. It is important at this time to note the extent of neck angulation and rotation of the juxtarenal aorta to optimize accurate endograft deployment. Broeders and Blankensteijn4 describe a simple technique to optimize imaging prior to endograft deployment. The juxtarenal aorta should be visualized in the sagittal plane to measure the degree of craniocaudad angulation (Figure 40-3). The axial sections are then used to measure the degree of aortic neck rotation (Figure 40-4). These angle calculations should be noted to ensure that the C-arm is orthogonal to the takeoff of the lowest renal artery. The tortuosity of the vessels, the degree of calcification and any thrombus is also noted. Circumferential thrombus or calcification will prevent complete apposition of the stent graft to the aortic wall and thus compromise the ability to achieve a proximal seal. Using 10% to 20% proximal graft oversizing, the stent-graft devices are typically ordered and delivered in advance of the scheduled case. The stent grafts are housed in relatively large sheaths and obviate either bilateral groin cutdowns or percutaneous access that is later closed with vascular closure devices. After introduction of the delivery system over a stiff wire to the juxtarenal aorta, an aortogram is performed, and under “roadmap” guidance, the main body of the stent graft is positioned and deployed just under the lowest renal artery. Care is taken to insert and deploy the main body of the graft with the contralateral gate deployed in an anterolateral position, which is optimal for subsequent retrograde cannulation. Once the contralateral gate is deployed, it is cannulated, and efforts are made to ensure that the catheter is indeed within the stent graft and not behind the graft in the aneurysm sac. When deploying devices with separate suprarenal fixation, a magnified aortogram is performed at this time to confirm the location and position of the renal arteries. Cranial obliquity is usually required to ensure that the origin of the lowest renal artery is seen in an orthogonal orientation. Once the suprarenal stent is deployed, the stiff wire is placed up the contralateral limb and a pelvic arteriogram with the C-arm obliqued 15 to 20 degrees to the other side is performed to demonstrate the origin of the contralateral hypogastric artery. The contralateral iliac limb is then deployed taking care to preserve flow to the internal iliac artery. The remainder of the main body or ipsilateral limb is deployed. In an obligate three-piece system, another pelvic arteriogram is performed in the opposite obliquity to demonstrate the takeoff of the ipsilateral internal iliac artery. The junctions are ballooned, and stiff wires are removed before the completion of arteriography to ensure that limb kinking is not a concern. Graft limb kinking is of particular concern in individuals with external iliac landing because of concomitant iliac artery aneurysms or small iliac artery diameters. Any type I or III endoleaks should be addressed in the operating room. Type I endoleaks can be addressed with proximal extensions, large-diameter bare stents, or simple ballooning. Type III endoleaks can be addressed with bridging pieces or repeated ballooning. Type II endoleaks can be observed, as the majority of them resolve with conservative management within 6 to 12 months. Completion arteriography should demonstrate both renal and hypogastric artery patency and good flow down both iliac limbs. In the setting of small calcified aortic bifurcations (<20 mm diameter), adjunctive bilateral kissing balloon angioplasty should be a consideration.
Endovascular Repair of Common Iliac Artery Aneurysms
The majority of individuals with common iliac artery aneurysms have concomitant abdominal aortic aneurysms. If the common iliac artery aneurysm encroaches on the aortic bifurcation such that there is not enough length for a proximal seal, or if they have a concomitant AAA, endovascular aortic stent-grafting is performed simultaneously. Intervention for common iliac artery aneurysms typically occurs when they are 3 to 4 cm in diameter. Endovascular repair involves coil embolization of the ipsilateral hypogastric artery to prevent a type II endoleak. The iliac limb is then deployed into the external iliac artery. Small series have shown that staging embolization of the internal iliac artery in the instances where patients have bilateral common iliac artery aneurysms is better tolerated and results in a lower risk of pelvic ischemia (i.e., colorectal ischemia, buttock claudication, and sexual impotence).5,6 Iliac branched devices have been developed and are currently under trial investigation in the United States, although initial results abroad are promising.7 The branched devices allow for preservation of flow to the internal iliac while achieving a distal seal in the external iliac artery. This study reported a secondary patency rate of 87.3% at 22 months.