Chapter 20 Endovascular Repair of Extracranial Cerebrovascular Lesions
Each year in the United States, nearly 800,000 individuals experience a new or recurrent stroke, accounting for direct and indirect costs of more than $40 billion and untold damage to patients and their families. Stroke is responsible for 1 out of every 18 deaths in the United States, making it the number three cause of death after heart disease and cancer.1 Carotid bifurcation stenosis and resultant ischemia producing emboli is a major cause of preventable stroke. Efforts to address this major health issue have focused on risk factor management, including medical therapy and surgical or endovascular intervention.
Treatment of carotid disease has evolved considerably over the past 60 years with the introduction of the carotid endarterectomy (CEA) in the 1950s and carotid angioplasty in the late 1970s. During the 1990s several landmark, randomized controlled trials (RCTs) in North America and Europe were published that demonstrated the superiority of CEA plus medical management over medical management alone, affirming CEA as the gold standard for patients with carotid bifurcation stenosis.2–9 Carotid stenting was introduced in the mid 1990s and has evolved with advances in technique, equipment, operator experience, and the routine use of cerebral protection devices. Carotid angioplasty and stenting (CAS) is a viable minimally invasive alternative to CEA for select patient populations and is continuing to develop as an option for treating carotid bifurcation stenosis. The purpose of this chapter is to describe the technique of CAS, provide an overview of current results, and offer perspective as to the value of this treatment in the management of extracranial cerebrovascular disease.
Technique
Patient Selection for Carotid Angioplasty and Stenting
Part of the motivation in the development of CAS was to address the needs of patients at high risk for CEA in an attempt to improve results of carotid revascularization. In the United States, the Centers for Medicare and Medicaid Services (CMS) has established criteria for patient eligibility for endovascular carotid interventions on the basis of being high risk for CEA, identifying both physiologic and anatomic factors making the patient a high-risk candidate for an open surgical procedure (Box 20-1). The use of CAS for patients at high risk for CEA is now an established practice. The rate of perioperative myocardial infarction in the CREST Trial (detailed later in this chapter) was about twice as high after CEA than after CAS. It may be that going forward, patients with any significant history of coronary artery disease are considered to be at increased risk for CEA and may be better served with CAS.
Box 20-1 Centers for Medicare and Medicaid Services Criteria
Patients at High Risk for Carotid Endarterectomy
• Left ventricle ejection fraction <30%
• Contralateral carotid occlusion
• New York Heart Association class III or IV
• Unstable angina: Canadian Cardiovascular Society class III or IV
• Renal failure: end stage renal disease on dialysis
• Common carotid artery lesions below clavicle
• Clinically significant cardiac disease (congestive heart failure, abnormal stress test, or need for open-heart surgery)
• High cervical internal carotid artery lesion
• Restenosis of prior carotid endarterectomy
From http://www.cms.org. Accessed May 14, 2011.
However, there are also factors that make patients high risk for CAS; these are primarily anatomic. Examples of poor anatomy for CAS include: severe tortuosity of the aortic arch, great vessels or carotid bifurcation, heavy calcification of these vessels, or access problems. Marked angulation, kinks, and coils of the internal carotid artery might not allow adequate room for appropriate deployment of cerebral protection devices (CPDs) with adequate wall apposition.10 If these areas are straightened by stents, the angulation is usually displaced distally and may be more exaggerated. Difficult arch anatomy or severe calcification of the aortic arch and proximal branch vessels may preclude a safe carotid intervention. Carotid bifurcation lesions that are recently symptomatic or are composed of soft plaque should also be avoided, if possible. These lesions can also be treated using proximal protection, so that the cerebral circulation is protected from emboli before crossing the lesion. Carotid bifurcation lesions that are too long to treat with a single stent also tend to add risk to the CAS procedure. Poor early results of CAS in older patients have prompted a high level of caution in octogenarians. Extra evaluation for high-risk anatomy, preexisting brain lesions, evidence of cognitive problems, or other factors that may make repair more risky or limit its long-term value should be performed. In the early phases of CAS, these high-risk factors for performing CAS were not yet established. It is now well recognized that CAS in its current form and with existing technology is not a direct replacement product for CEA. The results of CAS have improved steadily over the past 15 years. One of the reasons for this is improved patient selection based on a better understanding of which patients are at high risk for CAS.
Preprocedure Evaluation
Thorough evaluation and preparation of the patient before the procedure is essential for safe carotid intervention. The brachiocephalic anatomy is studied before the procedure to assess candidacy for the percutaneous approach. A thorough understanding of the arch, carotid, and cerebral arterial anatomy can be obtained with catheter-based arteriogram, computed tomographic (CT) angiogram, or magnetic resonance angiography. Several anatomic factors can be considered relative contraindications to CAS as mentioned previously. A National Institutes of Health (NIH) stroke scale or other objective evaluation is completed before to CAS. A CT or magnetic resonance image (MRI) of the brain is obtained in symptomatic patients and in those 80 years of age or older to evaluate for preprocedure cerebral pathology. Octogenarians have a higher risk of stroke with CAS; therefore it should be performed with caution.11 Antiplatelet therapy is administered; aspirin daily and clopidogrel (Plavix) 75 mg/day for 5 days before the procedure. In all cases, patients should have received clopidogrel (total dose of 300 mg) before the intervention. Antihypertensive medication is held or decreased on the day of the procedure. If antihypertensive is required during the procedure, it is best to use a short-acting agent. Postoperative hypotension or bradycardia can occur after CAS as a result of baroreceptor stimulation. Patients with aortic stenosis may have cardiovascular collapse in this setting, and external pacing pads or a temporary internal pacemaker should be placed. In patients with absent femoral pulses owing to aortoiliac occlusion, a transbrachial approach may be considered.
Arch Evaluation
Arch manipulation carries a risk of neurologic events. In several studies of CAS, up to 1% of patients sustained a stroke in the contralateral hemisphere, suggesting that carotid access is a contributor to morbidity.12,13 Administer systemic heparin before aortic arch manipulation. An ACT of 250 seconds or greater is desired.
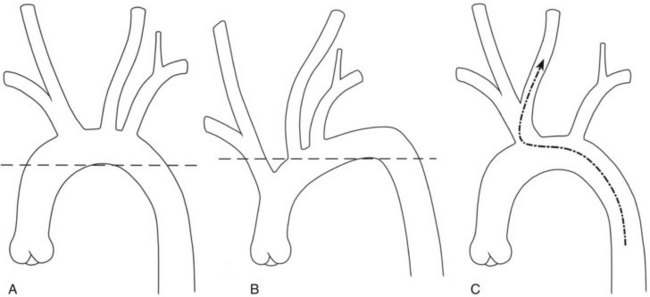
Hypertension and advanced age are associated with increased tortuosity of the access pathway to the carotid bifurcation. This makes no difference in the performance of CEA, but directly influences the challenges posed for CAS. Negotiating the tortuous arch requires more manipulation for catheterization, a more embedded position of the exchange guidewire, and more maneuvers to achieve sheath placement. The tortuosity of the arch can be assessed rapidly by drawing a horizontal line across the apex of the inner curvature of the arch.14 Vessels that originate below the horizontal line at the apex of the aortic arch (e.g., branches that arise from the ascending aorta) are more difficult to selectively cannulate (Figure 20-1). The authors caution against carotid stenting in the setting of a “difficult arch” until the operator has become expert with selective cannulation of the common carotid arteries in this situation. Even then, the tortuous arch likely poses a slight increase in the overall risk of CAS. Training and credentialing documents suggest varying numbers of carotid arteriograms as a prerequisite to initiating CAS training.15,16
Selective Common Carotid Cannulation
Complex curve or reversed-angle catheters such as the Vitek or VTK are usually required when the aortic arch is tortuous, the common carotid arteries are retroflexed toward the patient’s left, or there is a bovine arch configuration (Figure 20-2). Complex curve catheters are best reformed in the proximal descending aorta and then pushed proximally into the arch. The catheter is advanced with the tip angled anteriorly and then the tip is angled superiorly as the branch of choice for catheterization is approached. After the tip is engaged into the common carotid artery of choice, the catheter is adjusted slightly, usually with a gentle pull, to allow the elbow of the catheter (located at the second curve of a reversed curve or complex cure catheter) to reach its optimal intended configuration and seed itself in the artery origin. Arteriography can be performed from this position, and the catheter is unlikely to slip out. However, reversed-angle catheters cannot be as easily advanced into the branch vessels after cannulation of the origin. They are used mainly to access the origin of the branch vessels for a selective angiogram of the carotid arteries. Advancing the reverse curve catheter into the external carotid artery requires as much wire as possible be placed beyond the catheter tip. Because of the reverse angle, a forward push on the catheter shaft when it does not have a reasonably robust rail of wire to pass along will advance the catheter further proximally into the aortic arch and actually drag the tip out of the common carotid artery.
Carotid Sheath Access
Carotid sheath access requires placement of an adequate length of exchange guidewire into the common carotid artery. When the arch is straightforward and there is no tortuosity and the common carotid artery is of adequate length, this can be accomplished by placing the tip of the exchange guidewire in the distal common carotid artery. However, usually to place an adequate length of exchange guidewire, the external carotid artery must be catheterized and used to anchor the stiff guidewire (Figure 20-3). Selective external carotid cannulation can be accomplished with a 260-cm angled guidewire and the vertebral catheter. An attempt should be made to reach as distal as possible in the external carotid artery. This allows adequate guidewire length for the subsequent placement of the carotid sheath. Passage of the stiff exchange guidewire into the small external carotid artery branches must be done with caution to avoid injury or perforation to these branches. CAS can usually be accomplished with a 6- or 7-French sheath. The guidewire is then withdrawn from the vertebral catheter, and a 260-cm Amplatz Super Stiff or other exchange guidewire is passed into the external carotid artery. Caution should be used during wire exchanges. Removing the wire too quickly can create a vacuum, resulting in introduction of air emboli. It is sometimes helpful to administer contrast into the catheter to confirm external carotid placement. Contrast injections into the carotid system should not be done unless free backflow of blood is present at the hub of the diagnostic catheter. Otherwise there is a risk of pushing microbubbles into the system. In the external carotid artery, back bleeding can at times be diminished by the tight fit of the catheter in the small external carotid artery branches. In this event, the cerebral catheter is slowly withdrawn until adequate backflow is noted.
Cerebral Protection
There are no large randomized trials comparing CAS with and without cerebral protection devices, although their use has become standard of practice after early data suggested a decrease in embolic complications.10 The CMS also mandates CPD use for endovascular carotid interventions. There are numerous commercially available CPDs (Box 20-2) that are categorized as follows: distal filter, distal occlusion balloon, and proximal occlusion with or without reversal of flow.
Distal filter placement is by far the most commonly used CPD. The filter is placed distal to the lesion before stent placement, typically on a 0.014-inch guidewire system. These devices vary in shape and deployment, but once in place, they permit continuous antegrade flow into the intracranial internal carotid artery while serving as a barrier to embolic debris. Distal filters generally capture particles greater than 100 µm in size. Filters may be fixed to the wire or separate from the initial guidewire. The fixed-guidewire systems are simpler and require fewer steps. However, in the fixed-guidewire system, any guidewire movement after the filter is deployed results in movement of the filter itself. In addition, the attachment of the filter to the guidewire limits the handling of the guidewire during lesion traversal. The free-guidewire systems permit a choice of guidewires. When there is substantial tortuosity, a complex lesion, or other challenges, the free-guidewire systems have an advantage. The advantage of filters is that they allow continued cerebral perfusion, particularly in patients who have inadequate collateral circulation to permit temporary carotid occlusion (Box 20-3).