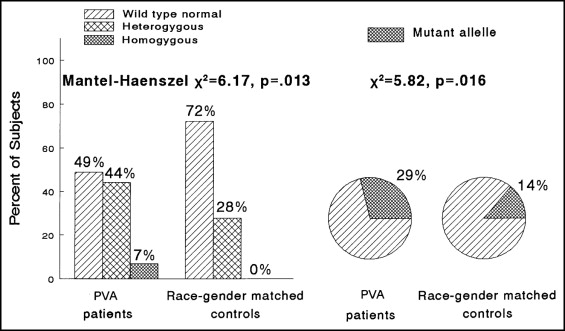
Because the endothelial nitric oxide synthase (eNOS) T-786C polymorphism is associated with reduced nitric oxide production and coronary artery spasm in Japanese patients, we speculated that it might be reversibly associated with Prinzmetal’s variant angina in white Americans. Polymerase chain reaction analyses of eNOS T-786C and stromelysin 5A6A polymorphisms were done in 31 women and 12 men (42 white and 1 black American, median age 50 years), with well-documented Prinzmetal’s variant angina. We matched each case with 1 healthy control by race and gender. Of the 43 cases, 21 (49%) were homozygous for wild-type normal eNOS, 19 (44%) were T-786C heterozygotes, and 3 (7%) were T-786C homozygotes. Of the 43 controls, 31 (72%) were homozygous for wild-type normal eNOS, 12 (28%) were T-786C heterozygotes, and 0 (0%) were T-786C homozygotes (p = .013). The mutant eNOS T-786C allele frequency in patients was 25 (29%) of 86 vs 12 (14%) of 86 in the controls (p = 0.016). Patients did not differ from controls for the distribution of the stromelysin 6A mutation (p = 0.66) or for the mutant 6A allele frequency (53% in cases, 50% in controls; p = 0.65). Nineteen patients took nitric oxide-elevating l -arginine (9.2 g/day, orally). Of these 19 patients, 10 (53%) became free of angina, 3 (16%) were improved but not angina free, and 6 (32%) had no change in their angina. Using l -arginine, the physical ability score (Seattle Angina Questionnaire) increased from a median of 42 to 72 of a total possible score of 100 (p = 0.011), satisfaction with symptom reduction increased from 53 to 61 (p = 0.004), and the perception of quality of life as acceptable increased from 29 to 50 (p = 0.001). In conclusion, the eNOS T-786C mutation appears to be a reversible etiology of Prinzmetal’s variant angina in white Americans whose angina might be ameliorated by l -arginine.
Because the endothelial nitric oxide synthase (eNOS) T-786C polymorphism is associated with reduced nitric oxide (NO) production and coronary artery spasm in Japanese and Brazilian white and African-Brazilian patients, we speculated that it might be reversibly associated with Prinzmetal’s variant angina pectoris (PVA) in white Americans. We also speculated that oral provision of over-the-counter, NO-elevating l -arginine (9.2 g/day) would ameliorate anginal symptoms in patients with PVA, particularly in T-786C homozygotes.
Methods
The present study was conducted according to the principles of the Declaration of Helsinki, and all patients provided written informed consent, following a protocol approved by the Jewish Hospital of Cincinnati institutional review board.
The polymerase chain reaction analyses were done at the Molecular Diagnostic Laboratory (Cincinnati, Ohio).
The patients were studied in the consecutive order of their referral by their cardiologists and physicians. The diagnosis of PVA was confirmed by reviewing the referring physician and hospital records. PVA was documented by coronary catheterization with evidence of coronary spasm with or without ergonovine in the absence of occlusive coronary artery atherosclerosis.
The patients were asked to complete the Seattle Angina Questionnaire at their first assessment ( Table 1 ) and every 3 months while taking 9.2 g/day oral l -arginine (Arginaid) ( Table 2 ).
| Category | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|
| Physical ability (dressing, walk indoors, showering, climbing, gardening, walking 1 block, running, lifting, sport) | 63 ± 27 | 60 | 17 | 100 |
| Symptom remission compared to 4 weeks earlier | 51 ± 26 | 50 | 0 | 100 |
| Angina frequency less during past 4 weeks | 56 ± 29 | 50 | 0 | 100 |
| Satisfied with treatment of symptoms | 49 ± 30 | 50 | 0 | 100 |
| Perception of overall quality-of-life status | 33 ± 25 | 33 | 0 | 92 |
| Category | Baseline | Follow-Up | p Value (pair Wilcoxon) | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Physical ability score | 54 ± 29 | 42 | 71 ± 25 | 72 | 0.011 |
| Symptom remission compared to 4 weeks earlier | 47 ± 15 | 50 | 60 ± 31 | 50 | 0.18 |
| Angina frequency less during past 4 weeks | 52 ± 27 | 50 | 66 ± 35 | 75 | 0.067 |
| Satisfaction with treatment of symptoms | 44 ± 29 | 53 | 64 ± 27 | 61 | 0.0036 |
| Perception score | 30 ± 25 | 29 | 52 ± 33 | 50 | 0.0013 |
⁎ Mean ± SD and median months of treatment with l -arginine (9.2 g/day) was 18 ± 13 and 14, respectively (interquartile range 5, 30).
In addition to the Seattle questionnaire, during follow-up, the patients were asked to grade their anginal symptoms during 9.2-g/day oral l -arginine therapy compared to their pretreatment baseline, with categories of complete resolution of angina, improvement in anginal pain without complete resolution, no change in pain, and worsening pain ( Table 3 ).
| Anginal Pain Relief | Patients (n) | eNOS Genotype | ||
|---|---|---|---|---|
| Homozygous for Wild-Type Normal eNOS | T-786C Heterozygous for Mutant Allele | T-786C Homozygous for Mutant Allele | ||
| Pain gone (n = 10) or better (n = 3) | 13 (68%) | 7 (54%) | 3 (23%) | 3 (23%) |
| Pain unchanged | 6 (32%) | 1 (17%) | 5 (83%) | 0 |
⁎ Mean ± SD and median months of treatment with l -arginine (9.2 g/day) was 18 ± 13 and 14, respectively (interquartile range 5, 30).
Information was obtained from all patients regarding their current drug therapy for PVA, current and previous smoking, exogenous estrogen use, and menopause status to facilitate the assessment of possible interactions between genotype and environment.
The patients were matched one to one with the controls by race and gender. The 43 controls were from a cohort of 72 healthy normal adults, including 40 previously described healthy adult hospital personnel, and 32 healthy adults evaluated during family studies of patients with hyperlipidemia. A detailed medical history was obtained for all 72 controls, who were entirely free of coronary heart disease and had never experienced angina.
As previously described, after an overnight fast, blood for polymerase chain reaction analysis was drawn in tubes containing ethylene diaminetetraacetic acid and the DNA was extracted for subsequent analysis of the 5A6A stromelysin and T-786C eNOS polymorphisms. DNA was isolated using the Capture Column (Gentra Systems, Minneapolis, Minnesota). Polymerase chain reaction measures of the 5A/6A stromelysin and T-786C eNOS were performed using previously published methods. The forward primer for the stromelysin polymorphism was 5-GGT TCT CCA TTC CTT TGA TGG GGG GAA AGA-3, the reverse primer was 5-CTT CCT GGA ATT CAC ATC ACT GCC ACC ACT-3. The patient DNA samples (100 ng) were denatured at 95°C for 1 minute, and then 33 cycles at 95°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute. The product was digested with TthIII according to the supplier’s instructions (New England Biolabs, Beverly, Massachusetts). The forward primer for the eNOS polymorphism was 5-TGG AGA GTG CTG GTG TAC CCA-3, the reverse primer was 5-GCC TCC ACC CCC ACC CTG TC-3. The patient DNA samples (100 ng) were denatured at 95°C for 5 minutes, and then 33 cycles at 95°C for 0.5 minute, 63°C for 0.5 minute, and 72°C for 0.5 minute. The product was digested with MSP I according to the supplier’s instructions (New England Biolabs).
The products of the polymerase chain reactions were then electrophoresed on a 10% polyacrylamide gel and the bands visualized using ethidium bromide.
All statistical analyses were done using Statistical Analysis Systems for Windows, version 9.1.3 (SAS Institute, Cary, North Carolina). The distributions of the stromelysin and eNOS genotypes were compared between the patients and gender- and race-matched controls (1 control for 1 case) using Mantel-Haenszel chi-square and chi-square analyses ( Figure 1 ). Odds ratios, with 95% confidence intervals, were calculated for heterozygous and homozygous versus wild-type normal eNOS between patients and controls for the eNOS genotypes.
The Seattle questionnaire scores at study entry and after a median of 14 months of 9.2-g/day oral l -arginine were compared using paired Wilcoxon tests ( Table 2 ).
The associations of eNOS T-786C genotypes with changes in anginal symptoms in 19 patients after a median of 14 months of oral therapy with 9.2 g/day l -arginine were assessed using chi-square analyses ( Table 3 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


