INDICATIONS/CONTRAINDICATIONS
As with other endoscopic reflux therapies, the ideal patients for TIF are those with minimal anatomic change at the gastroesophageal junction (GEJ) and moderate-to-severe GERD symptoms that are responsive to PPI therapy. TIF should not be performed in patients with significant hiatal hernias, severe esophagitis, esophageal dysmotility, or in the setting of esophageal varices. Also, TIF is not recommended for acid control in patients with Barrett’s esophagus because normalization of esophageal pH has not been consistently demonstrated.
Indications
 PPI-dependent patients with objective evidence of GERD
PPI-dependent patients with objective evidence of GERD
 Good response to PPI therapy
Good response to PPI therapy
 Medically fit for general anesthesia
Medically fit for general anesthesia
Contraindications
 Age <18 years
Age <18 years
 BMI >35 kg/m2
BMI >35 kg/m2
 Hiatal hernia >2 cm
Hiatal hernia >2 cm
 Esophageal varices
Esophageal varices
 Barrett’s esophagus
Barrett’s esophagus
 Severe esophagitis (LA class C or greater)
Severe esophagitis (LA class C or greater)
 Severe esophageal dysmotility
Severe esophageal dysmotility
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Patients with GERD may present with typical or atypical symptoms. Most often, GERD presents with heartburn and regurgitation and may progress to dysphagia and chest pain. Less commonly, patients may present with supraesophageal symptoms including chronic nausea, aspiration, asthma exacerbation, cough, hoarseness, and globus sensation, with or without typical GERD symptoms. Patients who have undergone a period of medical treatment with improvement of their symptoms with acid suppression may be candidates for TIF; however, further objective evaluation is required.
The preoperative evaluation prior to TIF centers on establishing objective evidence of acid reflux and assessing patient suitability for the procedure. At a minimum, patients should undergo an upper endoscopy to assess for the presence of hiatal hernia and esophagitis, and esophageal manometry should be performed to demonstrate adequate peristalsis. Ambulatory pH testing is useful for establishing the diagnosis of GERD in patients who do not have erosive esophagitis and those with atypical symptoms. Contrast radiography can assess the presence and size of hiatal hernia and a video esophagram can be used in lieu of manometry to establish evidence of normal esophageal motility.
Routine preoperative upper endoscopy with a rigorous assessment of the GEJ anatomy performed by the operating surgeon can improve patient selection for TIF. This allows for identification of reflux-related complications including severe esophagitis, strictures, and Barrett’s esophagus that may exclude TIF as a treatment option. Also, a detailed examination of the GEJ in the retroflexed view should be performed with the stomach sufficiently distended to produce effacement of the rugal folds. In this view, the crura can be seen impinging on the GEJ, and the transverse dimensions of the hiatus should be evaluated (Fig. 10.1). TIF should only be considered for patients in whom this measurement is less than twice the diameter of the endoscope because when the transverse hiatal diameter exceeds this threshold, the plication may fail due to a tendency to herniate into the thorax.
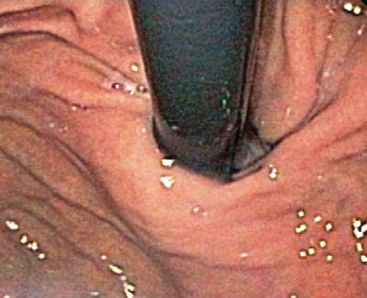
Figure 10.1 Retroflexion view of the gastroesophageal junction used to assess transverse hiatal diameter.
 SURGERY
SURGERY
Device
The EsophyX device (Endogastric solutions, Inc., Redmond, WA) is a gastroesophageal plicating device that is introduced into the stomach transorally over a standard endoscope (Fig. 10.2). This enables approximation of gastric and esophageal tissue in the region of the GEJ, and plicates these tissues together using placement of H-shaped full-thickness polypropylene fasteners.
The device consists of a handle (Fig. 10.3) where the device controls are located, a shaft that is 18 mm in diameter, and an articulating arm (the tissue mold) that approximates tissue and deploys tissue fasteners. When the tissue mold is placed in the retroflexion position, a tissue retractor with a helical screw at the tip can be advanced to engage and manipulate the mucosa at the GEJ (Fig. 10.4). A deployable stylet is located on either side of the helical retractor, and these serve to guide the deployment of the tissue fasteners (Fig. 10.5). The stylets are deployed through the esophageal and gastric walls and the H fasteners are deployed over the stylet so that the leading leg of the fastener engages in the gastric lumen, while the trailing leg remains within the esophageal lumen (Fig. 10.6).
Pertinent Anatomy
When describing the location of the fastener placements, it is useful to orient the retroflexion view of the GEJ as a clock face. The 12-o’clock position is defined as the location of the lesser curvature, with the 3-o’clock position on the anterior surface of the stomach and the 9-o’clock position on the posterior gastric wall. Six o’clock refers to the valve position that faces the greater curvature of the stomach (Fig. 10.7).

Figure 10.2 EsophyX device consisting of the handle, shaft, and articulating tissue mold. (© 2014 EndoGastric Solutions, Inc.)

Figure 10.3 EsophyX device handle. (© 2014 EndoGastric Solutions, Inc.)
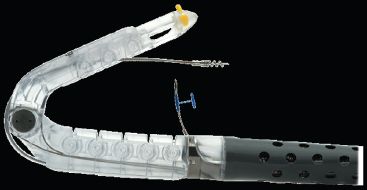
Figure 10.4 Partially closed tissue mold with deployed helical retractor. (© 2014 EndoGastric Solutions, Inc.)

Figure 10.5 Fully closed tissue mold with deployed stylet and polypropylene H fastener. (© 2014 EndoGastric Solutions, Inc.)
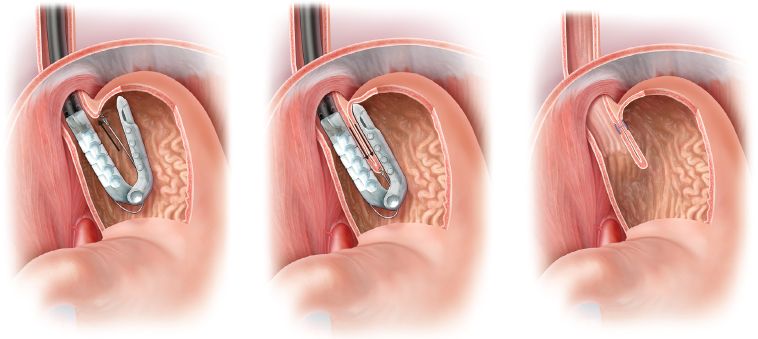
Figure 10.6 Schematic representation of tissue apposition and fastener deployment during TIF. Left: the helical retractor engages the mucosa at the level of the gastroesophageal junction. Middle: The tissue mold is closed to appose the esophagus and stomach while transmural fasteners are placed to recreate the angle of His. Right: Proper fastener position with leading and trailing limbs positioned within the stomach and esophagus, respectively.
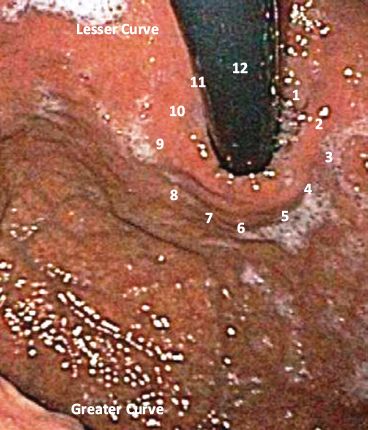
Figure 10.7 Preprocedure endoscopy shows a clear retroflexed view of the gastroesophageal junction demonstrating no evidence of hiatal hernia.
Technical Evolution
TIF is an endoscopic procedure that aims to create a gastroesophageal reflux valve through the creation of a full-thickness gastroesophageal plication. The technique used to create the plication has evolved since the introduction of the EsophyX device in 2007. The initial TIF-1 technique aimed to create the plication at the level of the GEJ, creating a partially circumferential gastrogastric fundoplication. The second iteration (TIF-2) involved creating a more robust gastroesophageal fundoplication by adding rotary and longitudinal elements and deploying fasteners 3 to 4 cm above the GEJ. This approach has proven capable of producing a 270-degree plication over a length of 3 to 4 cm. The most recent evolution of the TIF-2 procedure described here emphasizes the rotational elements of the gastroesophageal plication and achieving a valve of adequate length without including crural tissue in the plication.
Patient Positioning and Preparation
The patient is nasotracheally intubated and placed under general anesthesia. Nasotracheal intubation minimizes the space occupied within the oropharynx and facilitates passage of the EsophyX device into the esophagus. Sequential compression devices are placed, and preoperative antibiotics are administered due to the full thickness, transluminal application of the polypropylene fasteners. The patient is then placed in a semi-recumbent position with the left side elevated approximately 45 degrees. A preprocedure endoscopy is then performed to evaluate the GEJ anatomy and identify anatomical landmarks. Following this endoscopy, the hypopharynx and esophagus are dilated via placement of a 56-French Maloney dilator. Early in our experience, we did not use bougie dilation, but have since found that this greatly reduces the difficulties encountered with device insertion.
Technique
The device is generously lubricated and placed over a standard endoscope, and a bite block is placed over the device and between the patient’s teeth. The endoscope-device complex is inserted into the stomach transorally. A jaw-lift maneuver often facilitates easy passage of the device through the hypopharynx. Care must be taken during device insertion as hypopharyngeal perforations were reported during the early experience with this procedure. The endoscope is advanced into the stomach, a retroflexed view of the GEJ is obtained, and the stomach is insufflated with carbon dioxide to a pressure of 15 mm Hg using a standard high-flow laparoscopic insufflator. The device is advanced until the entire articulating arm is visualized within the gastric lumen (Fig. 10.8A). The endoscope is then backed up into the device (Fig. 10.8B) and the tissue mold is placed into the retroflexion position. Following this, the endoscope is advanced into the gastric lumen and retroflexed to view the GEJ and tissue mold (Fig. 10.9).
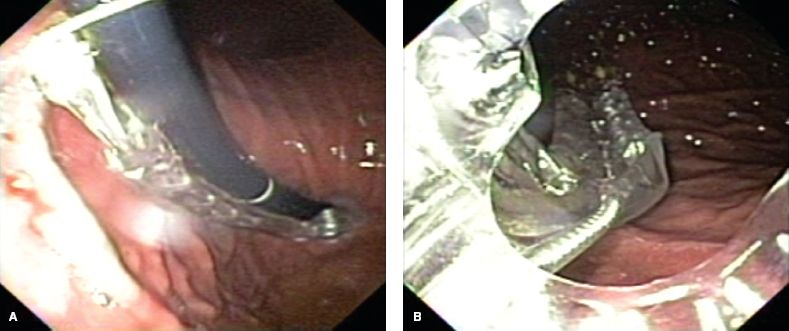
Figure 10.8 A: Retroflexed view of the device with the articulating arm completely advanced into the gastric lumen. B: Antegrade view from within the device of the articulating arm within the gastric lumen.



