Endoscopic Ablational Therapies and Stenting
Michael Zervos
Costas Bizekis
According to the American Cancer Society, there will be 215,020 new cases of lung cancer in 2008. This is the leading cause of cancer-related death in both men and women.1 Most patients with lung cancer will have advanced disease, and of these, a certain percentage will have an endobronchial component requiring some form of intervention. These patients may present with acute respiratory distress, worsening dyspnea, hemoptysis, or with pneumonitis resulting from endoluminal obstruction. Significant improvement in quality of life can be achieved if the airway obstruction is relieved.
In terms of lung cancer statistics as well as 5-year survival, the outcomes have not changed significantly over the past 10 years. Nevertheless, patients can be palliated with endobronchial obstruction and hemoptysis more efficiently and in a more durable fashion, because the treatment modalities and options have improved. Some of these are ablational, and all require some form of bronchoscopy whether flexible or rigid. These therapies include, for example, laser therapy cautery, rigid bronchoscopy, argon plasma coagulation, cryotherapy, photodynamic therapy (PDT), and stenting.2
Unfortunately, an almost equal percentage of patients that are diagnosed with locally advanced lung cancer present with metastatic disease, and of these, 20% will have an endobronchial component compounding their symptoms. Extrinsic or intrinsic luminal compromise eventually causes acute or chronic respiratory failure, and many patients will present with symptoms of hemoptysis, postobstruction pneumonia, or dyspnea.3
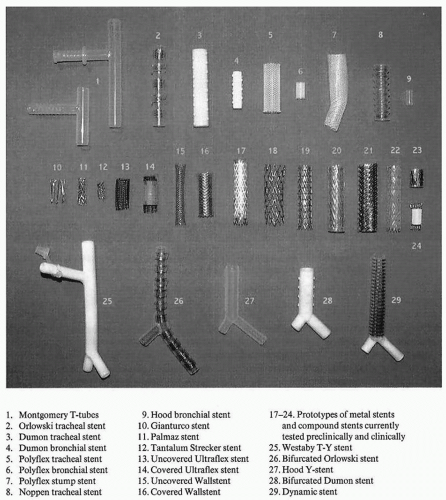
Palliation of bronchial obstruction frequently involves the use of a stent. “Stent” refers to a British dentist, Charles R. Stent, who created a mold for edentulous patients and since then has carried his name to describe any device that maintains the integrity of a hollow tubular structure. Originally, these stents were used in the gastrointestinal tract and in the vascular system and subsequently were applied to the airway. There are basically two types of stents: metal and silicone. Although they have essential differences with regard to their design and properties, they can both be used to palliate malignant central airway obstruction (CAO).
Currently, airway stents are either made of self-expanding metal for more permanent use or silicone if a temporary solution is needed. Complications of these stents include airway fracture with insertion or perforation (early or late), bleeding, granulation or tumor ingrowth, infection, migration, obstruction, as well as a small risk of death. There are no large randomized clinical trials to examine the utility of stent use in the patient with lung cancer. Limited case series from multiple institutions support the use of stenting techniques to help palliate these patients. Moreover, the palliation of symptoms by stenting is almost immediate in most cases and offers shortterm improvement in quality of life, with little or no effect on long-term and overall prognosis. Additionally, recent data supporting the use of therapeutic bronchoscopy in patients with non-small cell lung cancer can achieve not only palliation of their symptoms but also potential lung-sparing surgery.
HISTORICAL OVERVIEW
Although many techniques to treat and palliate CAO exist, the most current technique of rigid bronchoscopy with and without endobronchial laser was perfected in the early 1980s by Dumon et al.4 The key to this technique was the provision of superior airway control and the versatility of performing most endobronchial procedures. Subsequent to Dumon et al.‚s experience, there have been multiple series with large number of cases that demonstrate the efficacy of this modality. These include Cavaliere et al.5,6 in 1988 who published their experience with 1396 applications of rigid bronchoscopy with laser in 1000 patients, in 1994 with 2253 applications in 1585 patients, and in 1996 with 2610 treatments in 1838 patients. Venuta et al.7 published their series in 2002 with 273 patients with a goal of palliation or as a bridge to surgery. Most recently in 2006, Moghissi et al.8 published their data over a 21-year experience with 2235 treatments in 1159 patients. Their review implies that laser therapy of lung cancer still plays an important role in the palliation of inoperable cancer.8
Rigid bronchoscopy has withstood the test of time and with the combined use of either laser, cautery or just forceps alone remains the preferred modality with most airway interventionalists when dealing with CAO. Refinements in the protocol have, in some instances, led to improvement of results over laser and rigid bronchoscopy, and the use of electrocautery in the airway may be more cost-effective and readily available. Cautery can be easily delivered through the working channel of a specially designed therapeutic scope with a larger working channel. In a recent review of interventional techniques to treat malignant obstruction of the large airways, the authors imply that electrocautery is likely to replace laser as the preferred tool in these cases.9
Silicone stents have evolved over time from their initial description by Montgomery10 in 1965. The Montgomery tube is a silicone T tube placed through a tracheal stoma for the relief of subglottic tracheal stenosis or to support a reconstructed tracheal anastomosis (Fig. 61.1).10 Subsequently, the T tube was modified by Cooper et al.11 and by Duvall and Bauer12 in order to be placed endoscopically. Westaby et al.13 also modified the T tube into a bifurcating T-Y stent that could be pulled through a stoma.
Dumon14 in 1990 published his data on silicone stents with good results for airway obstruction. Typically, this stent has studs that are molded to the exterior to prevent migration and is deployed through rigid bronchoscopy. The Dumon silicone Y stent can be used for bilateral mainstem pathology as well as for tumors of the carina.
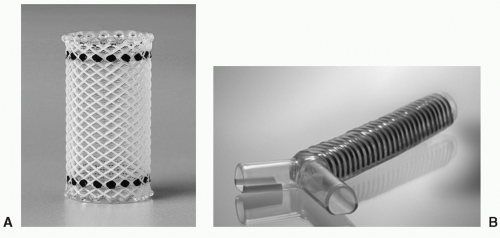
The most recent silicone stent that has been popularized by Boston Scientific is known as the Polyflex Airway Stent (Fig. 61.2A) This is a fully covered polyester silicone mesh that has its own delivery system with a loading basket and a pushing rod to facilitate deployment through a rigid bronchoscope. This can be used for all types of CAO regardless of etiology and has the obvious advantage of being removable. As with all silicone stents, mucus impaction and migration are the most common complications related to their use. Migration can be minimized with the airway Polyflex as long as the stent is appropriately sized with a tendency toward a larger diameter stent.
The Dynamic (Y) stent (Fig. 61.2B) is a removable airway stent made of silicone and has metal rings to resemble the cartilage of the trachea anteriorly. Also, it has a softer plastic posterior membrane that moves with respiration and resembles
that of the membranous trachea. Because of this design, it moves dynamically with each phase of the respiratory cycle. Placement requires use of special forceps also called Freitag forceps and can be positioned directly through the cords or with a special laryngoscope. A video laryngoscope may be helpful to visualize the cords to facilitate placement. This is indicated for CAO involving the carina, proximal mainstem, as well midto-distal tracheal pathology.15 More proximal lesions require a T-Y Montgomery tube with a tracheal stoma.
that of the membranous trachea. Because of this design, it moves dynamically with each phase of the respiratory cycle. Placement requires use of special forceps also called Freitag forceps and can be positioned directly through the cords or with a special laryngoscope. A video laryngoscope may be helpful to visualize the cords to facilitate placement. This is indicated for CAO involving the carina, proximal mainstem, as well midto-distal tracheal pathology.15 More proximal lesions require a T-Y Montgomery tube with a tracheal stoma.
Metal stents are also used to palliate malignant CAO. These stents can be deployed through flexible bronchoscopy, a guide wire, and fluoroscopy. Their sizes vary according to the desired length and diameter, and the stents are usually contained within a deployment system, which is released by pulling of a string that unravels and deploys the stent. Most of these stents have markers and can be visualized under fluoroscopic guidance.
EARLY METAL AIRWAY STENTS
The Gianturco stent was one of the first stainless steel metal stents with a zigzag loop design.16,17,18 This model is made of 0.018 inch stainless steel monofilaments into a double-zigzag design and is introduced via 12 French Teflon sheath. Palmaz was next to follow, which was positioned by balloon expansion and conformed to the airway. This feature prevents the stent from opening to a diameter larger than that of the given airway obstruction.16 These stents are rarely used in clinical practice in the United States today. Wallstent is a self-expandable super alloy stent that expands to a preset diameter and is mostly used in Europe.17,18 It is composed of 20 surgical steel monofilaments of 100-μm diameter braided into a cylindrical tube. It is mounted on a 7 to 9 French delivery catheter and a rolling membrane that serves to protect the device.
NEXT GENERATION METAL AIRWAY STENTS
Ultraflex (Boston Scientific) stents are made of single-strand nitinol.19 Nitinol is a memory metal and has the advantage of conforming to the irregularities of the airway (Fig. 61.3). This is a self-expanding stent that is contained within a flexible deployment system and unravels with the pulling of a thread. The deployment system can be passed over a guidewire that has been previously placed with a flexible bronchoscope or through an endotracheal tube. This obviously has the benefit of easy deployment and can be visualized under fluoroscopic control. The Ultraflex stent has upper and lower markers for accurate positioning across the obstruction. In addition to the noncovered version, this stent comes with a polyurethane cover that spans its length except for 1 cm on each end to allow for granulation and prevent migration. In a recent publication on Ultraflex stents, it demonstrates a low-complication rate and effective use in complex malignant airway stenoses with marked asymmetry or irregularity, angulation, or changing diameters.20
Included within the next generation of airway stent is the Aero Pulmonary stent by Alveolus company. This stent contains characteristics of metal and silicone stents. It has a biologically inert cover and can be removed if necessary. This stent can be deployed with either flexible or rigid bronchoscopy and can be repositioned after deployment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree