Endoluminal Management of Malignant Airway Disease
Joseph LoCicero III
Because patients with endobronchial tumor obstruction often present with acute and dramatic symptoms, they are the subject of continued investigation. Many physicians and surgeons developed innovative methods to relieve the acute symptoms. Now, most patients present with symptoms of obstruction after some initial therapy for lung cancer. Yet approximately 7% of all newly diagnosed lung cancers still present with primary obstruction.
Despite presentation with dyspnea, hemoptysis, or obstructive pneumonia, most patients can be managed conservatively. Hospitalization and initiation of oxygen therapy, sedation, and antibiotics often ameliorate the acute symptoms, allowing completion of medical evaluation and staging of the tumor. Approximately 20% of patients with newly discovered lung cancer still have early-stage disease and will be candidates for surgical resection. In such patients, pulmonary function testing, nuclear perfusion scanning, and accurate surgical mediastinal exploration may be necessary. The best form of treatment is palliative external beam radiation therapy for most of the late-stage or medically inoperable patients who have had no previous therapy. Endobronchial management thus becomes a secondary palliative method for those patients who remain acutely ill with initial unabated symptoms or those who fail following or progress during palliative radiation therapy.
Methods of Evaluation
Several investigators have attempted to objectify pretreatment and posttreatment signs and symptoms (Table 113-1). Quantitative signs and symptoms include segmental, lobar or lung collapse evident on radiography of the chest, changes in spirometry or flow-volume loop on pulmonary function testing, and changes in oxygenation status. Bronchoscopic documentation of percentage of obstruction is the most objective value and is the easiest to estimate. Semiquantitative signs and symptoms include shortness of breath, hemoptysis, and fever. Subjective criteria include nebulous symptoms such as fatigue, cough, wheezing, and stridor.
According to Schray and colleagues,28 68% of patients present with dyspnea, 37% with hemoptysis, 28% with cough without hemoptysis, and 14% with no subjective symptoms. Walsh and associates32 attempted to quantify the symptoms for better pre- and posttherapy comparisons. They used a dyspnea scale, evaluation of segmental collapse on chest radiography, pulmonary function testing including forced expiratory volume in 1 second, the ratio of this value to forced vital capacity, midexpiratory flow rate, and arterial oxygenation. They added a 6-minute walk test performed on level ground to assess the endurance of the patients and their potential for oxygen desaturation.
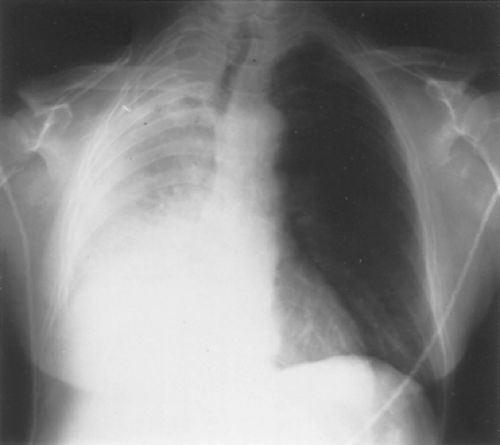
Despite careful evaluation, the author in 199917 confirmed that functional tests correlate poorly with symptomatic improvement. Most patients achieve symptomatic improvement with little change in objective numbers with the exception of the chest radiography. Radiographic evaluation of improvement in collapse also gives an excellent and easily evaluable criterion (Fig. 113-1). Peripheral saturation and the radiographic picture together are excellent predictors of the potential effectiveness of the therapy, because oxygen desaturation is a reflection of ventilation/perfusion mismatch due to segmental collapse. Cough and hemoptysis are present in many patients and are often relieved by noninvasive therapy. Improvement in the 6-minute walk test is usually but not always accompanied by symptomatic relief.
Another attempt at objective evaluation is the simple dyspnea scale (Table 113-2). This takes the subjective symptom of shortness of breath and makes it quantifiable. Some investigators add a general functional evaluation, such as the physical status scale (0 to 4) or Karnofsky’s scale (0 to 100), to their pretherapy evaluation.
Management Strategies
Surgeons must be familiar and facile with the wide variety of techniques available to manage tumor obstruction of the trachea and bronchi. Each has advantages and disadvantages. Sometimes a combination of techniques may be used to minimize the disadvantages of any one therapy. Patients who are quite symptomatic may be placed on a helium–oxygen mixture at either a 50%–50% or 70%–30% mixture. Because helium is a smaller molecule, it can move rapidly through tighter obstructions, delivering the mixed oxygen much more effectively than would be possible with nitrogen and oxygen (air). Heliox can provide dramatic short-term relief.
Table 113-1 Measures of Obstruction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Mechanical Debridement
The earliest method of endobronchial tumor management was simple mechanical removal. Jackson and Kozelman16 reported reasonable success with this technique in 1943. Later, in 1959, Jackson15 reported establishment of a patent airway simply by mechanical debridement, noting that with the use of a large rigid bronchoscope and adequate suctioning, many tumors could be debrided with minimal airway compromise. He noted that tumors of this size lack much vascularity, and that bleeding could be controlled through patience and persistence. Despite these reports, most tumor excision is combined with some method for the control of hemorrhage.
Electrosurgery
Endoscopic electrosurgery has been used safely for many years. Its principal use has been in removing colon polyps. More recently, it has achieved widespread use in the fulguration of various bleeding lesions of the upper gastrointestinal tract. It has been one of the resources of the thoracic surgeon for removing obstructing lesions from the airways, but few major reports of its use have been published.
Table 113-2 Dyspnea Scale | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hooper and Jackson13 cited eight previously reported cases and added four of their own. Methodology was rather straightforward and involved passing a probe through the aspiration port of the bronchoscope. They found cautery sectioning most easily accomplished for removal of a polypoid lesion. The alternate approach is vaporization using the closed wire snare as a cautery probe. A number of investigators have used the bipolar cautery adapted from the gastrointestinal laboratory with success.
Cryosurgery
Cryosurgery involves the use of a low-temperature probe that induces coagulation necrosis, which is generally limited to the immediate area of the probe. Liquid nitrogen is fed into a cannula that has an inner tube and an outer tube attached to a specifically designed interchangeable tip. Except for the tip, the cannula is insulated. Liquid nitrogen is fed under pressure and delivered to the tip, where it expands and escapes through the outer tube. Profound cooling occurs at the tip because of rapid gas expansion. Intracellular and extracellular formation of ice crystals injures cells and reduces their survival by disrupting membranes. This has a profound effect on decreasing blood supply.
In animal experiments laying the groundwork for this therapy, Carpenter and associates1 noted complete necrosis of the mucosa and thrombosis of vessels, with relatively well-preserved basic bronchial architecture. Surrounding areas of necrosis within the lung itself subsequently healed by fibrosis. The bronchi appeared to heal without stricture. The investigators later applied the technique in eight patients, with good results.
In 1986, Homasson and associates12 used a cryoprobe through a rigid bronchoscope for 27 patients. The cryoprobe itself was flexible and could reach into the right-upper-lobe bronchus. For malignant tumors airway palliation was achieved in 13 of 21 patients. Homasson and coworkers also easily ablated five small granulomas without complications. They recommended using flexible endoscopy 4 to 6 weeks after cryotherapy to aspirate and remove the slough, noting that because it took several days for necrosis to occur, immediate palliation of the airway was not obtained. With more experience, in 1996, another group headed by Mathur19 was able to completely remove the obstructions in 18 (90%) of 20 patients. Now, miniaturized flexible probes can be used through a flexible bronchoscope. However, variable airway damage and uncontrolled swelling limit the usefulness of this technique.
Laser
Initial airway clearing procedures were done with the carbon dioxide laser. Today, this modality has minimal use in airway obstruction. When the Nd:YAG laser became available for clinical use, it was an excellent tool for the endobronchial management of tumor. It causes coagulation necrosis, which can control vascular lesions. It also produces a 4- to 5-mm zone of necrosis beyond the visualized destruction that the endoscopist observes, which allows debridement of avascular tissue immediately after treatment. The early disadvantages were the high level of power and the need for water cooling, which limited the location of the laser. These problems have now been addressed and resolved.
In 1984, Dumon and colleagues8 emphasized some important safety features. These included low laser power levels (below 50 W), short pulses (under 3 seconds), and a maximum inspiratory oxygen content of 50%. They advised mechanical removal of necrotic debris as early as possible to reestablish the airway.
Cavaliere and colleagues3 published the largest recent series in 1996. They reported on 3,069 procedures on 2,008 patients. They used the rigid bronchoscope for 96% of the procedures. They used the laser in 91% of the patients, with an average of 1.4 applications per case (mean time between procedures, 102 days). They also used other modalities such as brachytherapy (3.3% of cases) and stents (19.5% of cases), with good results. Early mortality was an acceptable 0.4%. Although rigid instrumentation was used in this series, many endoscopists use flexible bronchoscopes for all but the most proximal lesions. However, airway stents are rapidly supplanting this modality.
Endobronchial Brachytherapy
Henschke,9 as early as 1958, described interstitial radiation therapy with permanently implantable sources for unresectable or residual lung cancer. In 1979, Hilaris and colleagues11 began endobronchial implantation. The major advance in this field came with the refinement of flexible systems that could be placed in the patient using flexible bronchoscopy. Mittal and associates22 placed a catheter into which radon, gold, or other radioactive sources could be injected for therapy. Patients tolerated these catheters well during the 3 to 5 days required for treatment.
The next advancement came with the ultrafast, high-dose remote afterloading techniques using iridium-192. Miller and Phillips21 described this technique in 1990. They used the Nucleotron (Leersum, The Netherlands) selectron high-dose system, which is a three-channel remote afterloading system. It consists of a treatment unit that stores the sources within a lead-shielded safe and can be controlled remotely. The unit can store 100 sources, which can be individually programmed for different treatment times. These sources are automatically corrected for source decay. The treatment time and the number and positions of sources can be programmed independently for each channel to give the required dose distribution for a particular patient or tumor geometry. The sources are placed into a 5 Fr catheter. The dose levels within 1 cm of the source range from 30 to 60 Gy.
Table 113-3 shows the results with this technique. In comparison with the Nd:YAG laser in a large series of patients, endobronchial brachytherapy showed equal survival, with similar symptom palliation. If the laser was used to open the airway to allow better implantation of the seeds, the results were improved to 95% survival at 6 months. Morris and associates23 updated the series in 2002. In 121 patients, they achieved early success in 72%.
Table 113-3 Survival Comparison of Treatment With Neodymium: Yttrium-Aluminum-Garnet Laser and Endobronchial Brachytherapy | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree