Fig. 39.1
Schematic of a HeartMate SNAP-VE ventricular assist device system. One can see the internal connections at the apex of the left ventricle where there is inflow into the device and outflow connected directly to the aorta. This system utilizes an external battery pack and controller system. LVAD left ventricular assist device
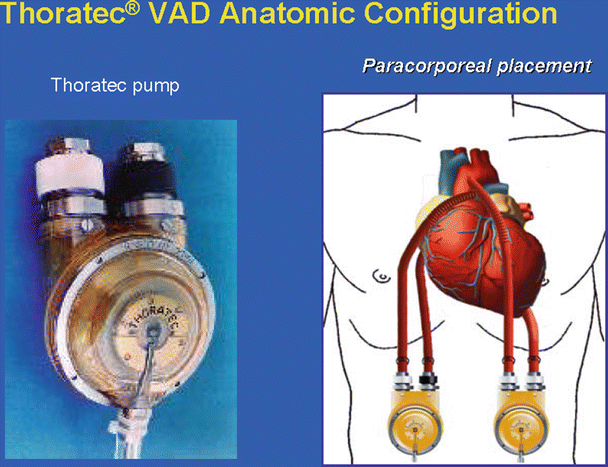
Fig. 39.2
Thoratec ventricular assist device (VAD). Left: pump with both inflow and outflow valve housing connectors pointing upward and the compressed air driveline pointing downward. Right: cannulas and grafts are implanted inside the body cavity, while the pumps are connected outside the body cavity
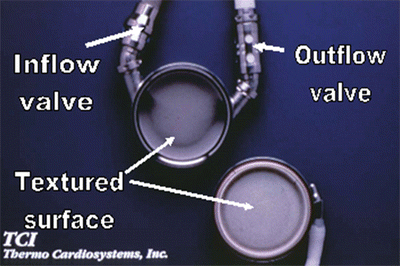
Fig. 39.3
Textured inner surface of HeartMate XVE left ventricular assist device. The textured surface promotes formation of pseudointima which subsequently prevents thrombosis formation
The HeartMate XVE LVAD is no longer used in the adult clinical setting, yet it played a very significant historical role in demonstrating that end-stage CHF patients could be successfully supported with an LVAD. When compared to maximal medical management including home inotropic therapy, the use of the HeartMate XVE LVAD significantly improved the quality of life and survival of end-stage CHF patients [5]. It is the first implantable LVAD that was approved as the destination therapy for end-stage heart failure in the USA. However, the disadvantages of this system include (1) its overall heavy weight, (2) its bulky size, and (3) frequent inflow valve and/or electrical motor failures, after an average of 12 months of implantation [3–5, 14, 15].
The Thoratec VAD [16] can be used as an LVAD, RVAD, or BiVAD. It is commonly described as an implanted paracorporeal pump, with the inflow cannula inserted in the left ventricle via the left ventricular apex and the outflow graft sewed to the ascending aorta when used as an LVAD (or the inflow cannula inserted in the right atrium and the outflow graft sewed to the pulmonary artery as an RVAD). This system allows for numerous cannulation options. Its pump is made of a flexible sac housed in a plastic ball-shaped container. Currently, the sac material is composed of Thoralon which has a smooth surface to reduce the risk of thrombosis. This sac is squeezed by the compressed air via an air-driven console, and it has two mechanical valves, one as an inflow and the other an outflow valve. Importantly, these units can be implanted in small- or large-size patients (weight between 17 and 144 kg) and can be readily employed for short-term as well as long-term support. To date, this device is approved by the FDA to be used as a bridge to heart transplant and as postcardiotomy support to recovery. The disadvantages of this system include the paracorporeal pump being attached outside the body, which can be inconvenient to patients, and patients require anticoagulation with coumadin to maintain an INR between 2.5 and 3.0 to prevent thromboembolism.
39.3.2 Continuous Flow Pumps: Axial Design
The axial flow pumps are typically regarded as second-generation VADs, as compared to the first generation of volume displacement pumps. Such pumps incorporate both continuous flow and rotary pump technologies as the foundation of their design and construction. They have an impeller inside the pump that can generate high-speed rotations in a blood system (Fig. 39.4). Depending on the motor capacity and rotational speed, an axial flow pump can function either as a partial-flow or full-flow support VAD. The speed of a rotor can reach an RPM of 9000, and it can generate up to 10 L/min of flow with a mean pressure of 100 mmHg.
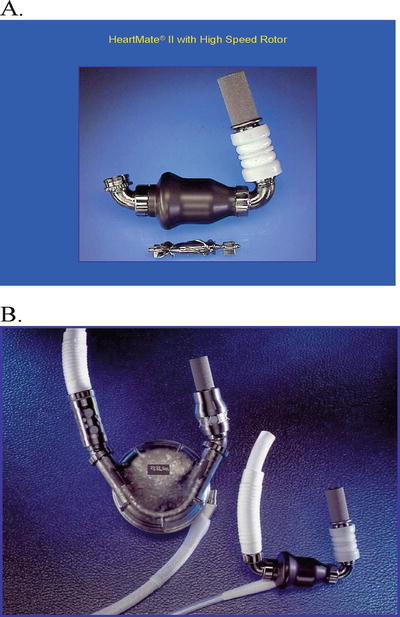

Fig. 39.4
HeartMate II left ventricular assist device. (A) HeartMate II pump and the high-speed rotor. The inflow cannula is connected to the pump. (B) Size comparison between the HeartMate VE and HeartMate II pumps. The inflow cannulas of both pumps are the same
Initially, serious doubts about the utility of such pumps were raised due to the concerns of red blood cell destruction and heat generated by the high-speed motor inside the blood system. But (starting with conjunction) such theoretical concerns were discarded when a Hemopump was successfully used in a clinical setting without significant hemolysis [17]. The Hemopump thus set the stage for evaluation of other types of axial flow pumps that could be used to pump blood in the human body [6, 7, 9–11].
The common features of axial flow pumps include the following: (1) the simpler design of continuous flow rotary pump technology promises increased long-term mechanical reliability; (2) they do not require valves to create a unidirectional flow nor do they require an external vent or a compliance chamber, thus making them more likely to be used as the platform for future development of a totally implantable VAD; (3) the inflow cannulas are inserted into the left ventricular apexes or totally inside the left ventricle, and the outflow grafts can be connected to either the ascending or descending thoracic aorta (i.e., which is important if abnormalities in the ascending aorta exist); (4) the size of such pumps is relatively small, one-fifth to one-seventh the size of the volume displacement pumps, thereby extending therapy to underserved patient populations including women and even some children; and/or (5) these pumps are associated with minimal noise generation and overall greater patient comfort [6, 7, 9–11, 14, 15].
The HeartMate II LVAD (Thoratec Corp.), the DeBakey MicroMed LVAD (MicroMed Cardiovascular, Inc., Houston, TX, USA), and Jarvik 2000 (Jarvik Heart, Inc., Manhattan, NY, USA) are currently available representatives of this group of VADs. Among them the HeartMate II and the DeBakey MicroMed LVAD have many similarities: (1) they are about the same size, (2) both devices consist of an internal blood pump with a percutaneous lead that connects the pump to an external system driver and power source, and (3) both pumps can generate up to 10 L/min of flow. The initial results appeared to be encouraging, with data supporting effective circulatory support and durability [6, 7, 9–11, 14, 15]. Of particular interest is that the diminished pulse pressures that are associated with utilizing an axial flow pump seemed to be tolerated well in patients, at least for the short term. Importantly, the long-term consequences of using axial flow pumps have yet to be evaluated. Additionally, hemolysis associated with the use of axial flow pumps has been detectable, yet the level has been considered clinically insignificant. Nevertheless, these pumps seem to activate platelets because of the physical strain on blood cells; such activation could be an important source of intravascular thrombosis and/or embolic complications. It should be noted that the incidence of thromboembolism seems to be particularly high for the MicroMed LVAD. Therefore, intense anticoagulation regimens targeting platelet activation and clotting cascade have been employed to deal with these potential problems. A combination of coumadin and Plavix has been recommended for patients who have been provided with a MicroMed LVAD.
The HeartMate II LVAD has shown improved clinical outcomes both as bridge to transplant and destination therapy, with markedly better functional status and quality of life. An actuarial survival of 89 % at 1 month and 75 % at 6 months was reported [14]. The HeartMate II LVAD has proven to be safe and effective as bridge to transplantation. Furthermore, compared to HeartMate XVE, the HeartMate II significantly reduced the device- and surgery-related complications. It is considered that the lower incidence of postoperative bleeding and device-related infection may be due to its smaller size and the lack of need for a large pocket to house its pump and smaller driveline. The absence of a large preperitoneal pocket (which was required with the larger pulsatile devices) has reduced: (1) the need for extensive dissection, (2) postoperative bleeding, and (3) LVAD pocket hematomas and the development of pocket infection. Based on multiple clinical trial outcomes [14, 15, 18], the FDA approved the HeartMate II LVAD to be used as bridge to transplant as well as destination therapy for end-stage CHF patients in 2008 and 2010, respectively. To date, the clinical use of the HeartMate II LVAD has increased rapidly since FDA approval, and thus far over 17,000 patients have received implantation of the HeartMate II LVAD worldwide.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


