CHAPTER 28 Empyema
An empyema is a collection of pus in a natural body cavity. One of the most common varieties of empyema is empyema thoracis, which can be localized (i.e., encapsulated) or can involve the entire pleural space.1 Empyema thoracis is defined as a purulent pleural effusion. Although this infection usually originates from the lung, it may enter through the chest wall, from below the diaphragm, or from the mediastinum. Complications from elective thoracic surgery or from post-traumatic hemithoraces are other possible causes. Most empyemas are, however, parapneumonic, and they occur when the host is overwhelmed by the number and virulence of the organisms in the inoculum. Whereas the normal pleural space is resistant to infection, the abnormal space, such as one containing air, blood, or other fluids, is highly susceptible to empyema formation. The therapy for empyema depends on the pathogenesis of the pleural infection. In this chapter, some of the most controversial issues concerning pathogenesis, diagnosis, and management of postpneumonic empyemas are addressed. The problems associated with post-traumatic or postoperative empyemas are covered extensively in other chapters.
HISTORICAL NOTES
Empyema of the pleural cavity was recognized approximately 2400 years ago, when Hippocrates made the distinction between empyema and hydrothorax.2 Hippocrates diagnosed an empyema on the basis of its clinical presentation. Fever was constant, but mild during the day and increased at night. The patient’s cough was nonproductive, the eyes were hollow, and the cheeks showed red spots. When the patient was shaken by the shoulders, splash succussion sounds could be heard from the thorax, depending on the presence and amount of air and fluid. In his book on chest auscultation, Laennec translated Hippocrates’ description that distinguished hydrothorax from empyema: “When applying the ear on the ribs, during a certain time you hear a noise like boiling wine gar, which suggests that the chest contains water and no pus.”3 Sometimes, the noises were not heard, depending on the quantity and physical characteristics of the intrathoracic liquid.
Hippocrates is also credited with the first drainage operation for empyema by using cautery or trephination of a rib. As reported by Paget, Hippocrates opened the chest where the pain and swelling were most evident.4 He packed the wound with a strip of linen cloth, which was changed every day. He observed that this packing allowed fluid to escape around the strip but prevented air from entering the space. Daily irrigations with “warm wine and oil” cleaned the lung surfaces, and when the empyema had healed, metal rods were used to close the wound. He clearly understood the natural history of undrained empyemas when he wrote in a treatise on pleurisy and peripneumonia, “Patients with pleurisy who, from the beginning, have sputum of different colors or consistencies die on the third or the fifth day, or they become suppurative by the eleventh day.”5 Hippocrates also wrote, “When empyemas are opened by the cautery or by the knife, and the pus flows pale and white, the patient survives, but if it is mixed with blood, muddy, and foul smelling, he will die.”
In the 19th century, aspiration of acute pleural effusions was introduced. Wyman and his colleague Bowditch are credited with establishing this procedure.6,7 Wyman described the first therapeutic thoracentesis in a letter addressed to Sir William Osler: “With Dr. Homans’ advice and assistance, the chest was punctured with an exploring trocar and cannula between the sixth and seventh ribs about six inches from the spine, and twenty ounces of straw colored serum drawn off slowly with great relief of the symptoms.” Needles used for pleural aspiration, cannulas, devices preventing the entry of air, and suctioning systems were developed during the 19th century.8
Thoracentesis was modified with a closed-tube thoracostomy, according to descriptions by Playfair9 and Hewitt,10 who performed drainage with a trocar and then placed a rubber tube through the cannula into the pleural space. The rubber drain was connected to a glass tube that went through a cork into a bottle with a sealing level of antiseptic solution. It acted like a unidirectional valve, allowing the liquid to leave the thoracic cavity but keeping air from entering the space. The sealing level could be adjusted depending on the type and amount of fluid being drained. This system constituted a true siphon drainage system that also allowed pleural irrigation. In 1891, von Bulau popularized the underwater drainage system throughout Europe. His name is still associated with this “no suction” method of pleural drainage.11
The consequences of open pneumothorax and the importance of closed-tube drainage were not truly appreciated until a clear understanding of the pathogenesis of pleural infection was provided by Graham and Bell.12 Prior to their report, acute empyemas were managed by rib resection and open drainage; unfortunately, mortality rates averaged 30%. Death frequently occurred within 30 minutes of the procedure and was attributed to the open pneumothorax and mediastinal instability rather than to the empyema itself. Soon after Graham and Bell recommended closed rather than open drainage to treat early empyemas, the mortality rates decreased to 5% to 10%.13,14 The principles of empyema management as described by Graham and Bell included (1) careful avoidance of open pneumothorax during the acute stage, (2) prevention of chronicity by rapid sterilization and obliteration of the space, and (3) careful attention to the patient’s nutritional status.
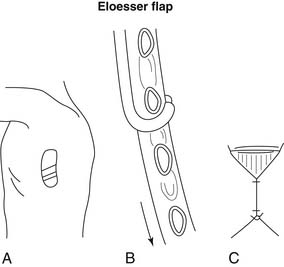
Open drainage is indicated only when fibrotic changes have occurred within the space. In 1935, Leo Eloesser described a tissue flap for the treatment of pleural tuberculosis. This flap was constructed as a one-way valve (Fig. 28-1), allowing the exit of pus but preventing the entry of air.15
As thoracic surgery evolved rapidly during the end of the 19th century, procedures such as thoracoplasty16,17 and decortication18,19 were introduced. These procedures described the obliteration of space either by collapsing the chest wall over the lung or by attempting to reexpand the lung itself. The results were not always good, but in 1901, Fowler stated that decortication was applicable to all patients with nontuberculous empyemas who could tolerate the procedure.20 He even said that “decortication could be used instead of Estlander’s thoracoplasty operation in most cases and should replace the Schede’s thoracoplasty altogether.” In 1923, Eggers reported on 146 patients who underwent decortication, and he described in full details the procedure as it is still used today.21
At the end of the 20th century, another modality was introduced for the diagnosis and treatment of empyema—video-assisted thoracic surgery (VATS), or thoracoscopy. Wakabashi first used thoracoscopy for the drainage of an empyema.22 Today, VATS is the modality of choice for the diagnosis and treatment of early empyema.23
With the onset of the antibiotic era, the incidence of pneumococcal and streptococcal empyemas fell sharply, and the mortality rate also declined dramatically. Subsequently, the increasing significance of anaerobic infection and the development of new generations of drug-resistant organisms have led to a new spectrum of problems. In addition, the number of patients with acquired immunodeficiency syndrome (AIDS) has increased, as has the number of patients receiving chemotherapy, and this has somewhat modified the natural history of empyema, because patients are no longer able to produce the inflammatory reaction that is fundamental to localizing the empyema and obliterating the space.24
STAGES OF PROGRESSION
The American Thoracic Society in 1962 divided the formation of an empyema into three distinct stages indicative of disease progression in the pleural space. Progression through these stages usually occurs over a 3- to 6-week period. During the exudative phase (stage I), the pleural membranes swell considerably and discharge a thin exudative fluid. Fibrin is deposited over all pleural surfaces and, despite early angioblastic and fibroblastic proliferation that extends outward from the pleura, the peel is not thickened enough to prevent complete lung reexpansion once the space is emptied. During the fibrinopurulent phase (stage II), fibrin is heavily deposited over all pleural surfaces—more over the parietal pleura than over the visceral pleura. The pleural fluid is turbid or frankly purulent and has a large number of polymorphonuclear white cells. At this stage, the pleura is still relatively intact, and the lung, although less mobile, can be reexpanded. Loculations form during this stage. Usually within 3 to 4 weeks, organization (stage III) begins, with massive ingrowth of fibroblasts and formation of collagen fibers over both parietal and visceral surfaces. The pus is very thick, and the lung, which is now virtually functionless, is imprisoned within a thick fibrous peel. The lung can no longer expand without being decorticated. Finally, arterioles infiltrate the peel within 6 weeks. In a 10-year retrospective analysis of 101 patients with empyema, Renner and colleagues found that 17 patients had stage I empyema, eight were in the purulent stage, and 76 (75%) had an organized empyema.25 (For management purposes, two stages are recognized: an acute process [stages I and II] and a chronic process phase [stage III].)
COMPLICATIONS
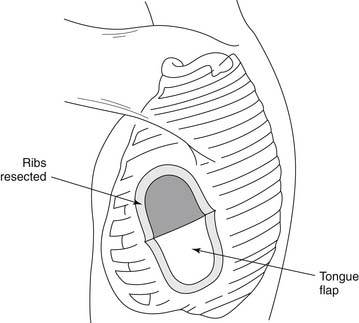
Complications can occur at any time during the formation of an empyema, but they are more likely to develop during the chronic stage of the disease process. One of the most common but often unrecognized complications is increased fibrosis and scar tissue in the lung, which produce pulmonary fibrosis. Scar tissue can also penetrate the parietal pleura and reach the intercostal spaces, which become narrowed and contracted, giving the chest wall the appearance of a carapace.1 This penetration can to lead severe pleuritic pain with ensuing shortness of breath. In extreme cases, the shape of the ribs is altered, and on cross section, they appear triangular. In other instances, calcifications may develop in the fibrous tissue, and bone may be formed. Empyema necessitatis is characterized by the dissection of pus through the soft tissues of the chest wall and eventually through the skin. Similarly, the sudden appearance of purulent sputum signals the development of a bronchopleural fistula with spontaneous drainage of pus into the bronchial tree (Fig. 28-2). In a series of 77 patients with bronchopleural fistula studied by Hankins and colleagues, spontaneous fistulas (n = 28) were secondary to tuberculosis in 23 patients and to bacterial pneumonia or lung abscess in five.26 Unusual complications include rib or spine osteomyelitis, pericarditis, mediastinal abscesses, or transdiaphragmatic drainage of the empyema into the peritoneal cavity.
Parapneumonic Effusions
Patients with bacterial pneumonia may have an associated pleural effusion, which is called a parapneumonic or postpneumonic effusion. Uncomplicated effusions are nonpurulent, are negative by Gram stain and by culture, and do not loculate in the pleural space. They resolve spontaneously with antibiotic treatment of the underlying pneumonia.27 Complicated effusions are either empyemas or loculated parapneumonic effusions that require surgical drainage for adequate resolution. According to Light and colleagues, the pH of the pleural fluid as well as its lactate dehydrogenase (LDH) and glucose levels appear to be useful to differentiate uncomplicated from complicated parapneumonic effusions.28
PATHOGENESIS
Most empyemas are the result of bacterial suppuration in organs that are contiguous with the pleural surface. Among these, the lungs are the most common source. In such cases, empyema occurs by direct bacterial spread across the visceral pleura or by free intrapleural rupture of microscopic and peripherally located lung abscesses. In a classic description of putrid empyemas, Maier and Grace showed that most were associated with bronchiectasis, pulmonary abscess, and suppurative pneumonia.29 In most series, empyemas are secondary to bronchopulmonary infections in 50% to 60% of cases and nearly all of the so-called primary empyemas result from subclinical pneumonic processes.20,30,31
In 1971, Vianna showed that several patients with postpneumonic empyemas had various underlying conditions, such as alcoholism or chronic pulmonary disease.32 Inactive pulmonary tuberculosis, diabetes mellitus, long-term steroid therapy, and various malignancies are other common predisposing conditions. Substance abusers and immunosuppressed individuals, such as patients with AIDS, are also at risk for bacterial and aspiration pneumonia and other pulmonary infections.33 These may lead to parenchymal destruction with subsequent contamination of the pleural space, which results in either simple empyema or complex infections including bronchopleural fistula.
Other potential sources of contamination should be sought when the cause of empyema is unclear. Rupture of the esophagus, for example, nearly always results in empyema formation. Rare causes of contamination, including infection in the deep posterior region of the neck and, less frequently, infections in the chest wall or thoracic spine. Although subphrenic abscesses can occasionally contaminate the pleural space through direct transdiaphragmatic erosion, most effusions associated with these abscesses are sterile exudates known as sympathetic effusions. LeRoux showed that lymph drainage from subphrenic spaces can travel cephalad through the diaphragm, and this is the likely route of transferal of subphrenic infections to the pleural space.34 He also noted that silent paracolic abscesses can occasionally erode through the diaphragm and infect the pleural space.
Virtually all post-traumatic empyemas are associated with penetration of the chest wall or the presence of a hemothorax. In a large series of trauma patients seen between 1972 and 1996, Mandal and Thadepalli reported a 1.6% incidence of empyema formation.35 In penetrating thoracic injuries, empyema formation is usually the result of organic foreign bodies being carried into the pleural space.36 In an interesting study, Ogilvie showed that the nature of the missile (e.g., shell splinters, bullets, or bayonets) played little part in determining the rate of infection in empyemas secondary to penetrating injuries.37
After blunt thoracic injury, hemothoraces become secondarily infected via contamination through the chest tube or from an infection in the adjacent lung. In 1977, Arom and colleagues made a distinction between post-traumatic empyemas and infected organizing hemothoraces (clotted hemothoraces) in which masses of blood clot became secondarily infected.38 Ogilvie showed that air in the pleural space that is associated with blood is more likely to get infected than is a pneumothorax or a hemothorax alone.37 In an experimental model for empyema thoracis, Mavroudis and colleagues showed that a concomitant hemothorax increased the incidence of empyema and early death after Staphylococcus was inoculated into the pleural space.39 In rarer cases, traumatic empyemas follow blunt esophageal rupture, acute diaphragmatic hernia with bowel strangulation or necrosis, or aspiration of a foreign body with perforation of the lung.40 Direct inoculation of the pleural space can occur as a result of minor thoracic interventions, such as thoracentesis, thoracic biopsies, or chest tube drainage when sterile technique is not followed.
Postoperative empyemas are seen almost exclusively after operations in which the esophageal or bronchial lumina have been entered. The incidence of this complication is in the range of 2% to 4% after pulmonary resection. In recent years, prophylactic use of antibiotics during the postoperative period and improved surgical technique have played a significant role in lowering the incidence of these events. There is limited evidence that hematogenous infection of the pleural space can occur from a distant infection site (classically, osteomyelitis) without an intermediate lung infection, which then contaminates the pleural space. In Sherman and colleagues’ series, only four cases represented true metastatic hematogenous seeding of the pleural space.31
Bacteriology
In the pre-antibiotic era, the predominant organisms recovered from empyemas were pneumococci and Streptococcus pneumoniae.41,42 In summarizing a total of 3000 empyemas reported from 1934 to 1939, Ehler noted that pneumococci were found in 64% of cases, Streptococcus pyogens in 9%, and Staphylococcus aureus in 7%.43 He concluded that other organisms were found rarely and should be considered curiosities. The incidence of empyema was greater (80%) with streptococcal pneumonia than with other types of pneumonia because of greater lung destruction associated with the causative organism. In those cases, myriads of tiny lung abscesses occurred along the lymphatic channels and discharged the infecting organisms into the effusion in great quantities; this converted the effusion into an empyema in a matter of hours.44
The introduction and increasing use of antibiotics was accompanied not only by a marked reduction in the incidence and mortality rates of empyemas but also by a change in the spectrum of causative organisms. In a study on the changing etiology of acute bacterial empyema, Finland and Barnes showed that although the incidence of streptococcal pneumonia generally declined from 1950 to 1953, it still continued to occur in community-acquired empyemas.45 The incidence of S. aureus–related empyemas increased, and it became the most frequently found organism in empyemas in 1955. It declined to its original levels after 1965, but gram-negative rods increased in importance.
The predominant isolates in recent years have been S. aureus (29% to 69% of culture-positive cases) and enteric gram-negative bacilli (29% to 60% of culture-positive cases).46 In a report by Vianna, 41 patients with bacterial pneumonia complicated by empyema were studied, and S. aureus was the most common causative organism isolated (34%).32 Gram-negative bacteria were isolated in 64% of empyemas that complicated some other underlying disease, probably as a result of previous antibiotic therapy. The incidence of S. aureus–induced empyema has also increased in children. From 1955 to 1958, it was the causative organism in 92% of cases in children younger than 2 years, as reported by Ravitch and Fein.47 In countries where the introduction of new antibiotics and new administration techniques was delayed, the changes in the bacteriology of empyemas were seen at a later date.
The recovery rates of anaerobes isolated from empyemas vary from 19% to 76%.46,48 These microorganisms are normal inhabitants of the mouth, intestine, and female genital tract. They reach the lung by aspiration from the mouth or bacteremic spread from the intestines or areas of pelvic suppuration. In a series reported by Sullivan and colleagues, 226 culture-proven empyemas were analyzed, and anaerobes were isolated from 44 patients.48 More than 50 anaerobic bacteria were identified, but the most common was Streptococcus.
In the series by Bartlett and colleagues, 76% of patients with empyemas had anaerobes either alone (35%) or in combination with aerobic agents.46 In most cases, the flora were complex, with an average of three different species of bacteria per case. According to these authors, the paucity of anaerobic isolates in most reports of empyema is related to the inadequacy of methods to preserve oxygen-sensitive forms during transfer to the laboratory and the lack of adequate anaerobic culture technique.
Often, a culture of empyema fluid does not establish a microbiologic diagnosis. In the series by LeRoux, a causative organism could not be isolated in 80% of patients.34 In other series, the percentage of negative cultures varied from 25% to 60%.49,50 In general, negative cultures result from inadequate culture techniques or very effective antibiotics that can penetrate the empyema and prevent bacterial growth. When empyema necessitatis occurs, the pathogens recovered do not necessarily represent the microorganism responsible for the disease, because the skin fistula may be contaminated with skin flora or hospital pathogens.49
DIAGNOSIS
In the series by Varkey and colleagues of 72 cases of empyema, the most common initial manifestations were dyspnea (82%), fever (81%), cough (70%), and chest pain (67%).51 In addition, a major underlying disease was present in 45 patients. Because the symptoms are related to the cause and stage of empyema, the amount of pus in the pleural space, the status of the host defense mechanisms, and the virulence of the microorganisms involved, patient experience may vary from a few symptoms to several, with severe toxicity. Symptoms may also vary with the cause of the empyema. Patients with parapneumonic empyemas, for example, often present with cough and purulent sputum, whereas the symptoms of patients with empyemas secondary to subphrenic abscesses may be exclusively abdominal complaints. On the basis of the clinical history, Maier and Grace divided cases of putrid anaerobic empyemas into two groups.29 In the first, expectoration of foul sputum indicated the presence of a pulmonary anaerobic process or of an anaerobic empyema with an associated bronchopleural fistula. In the second group, the foul sputum was absent, and the symptoms suggested ordinary pneumonia. Most patients with an empyema have leukocytosis with a shift of the cell count to the left.
Chest radiographs show a pleural effusion with or without underlying pneumonia or lung abscess. On lateral radiographs, the empyemas are nearly always posterior and lateral, and most extend to the diaphragm. The classic image is that of a posteriorly located, inverted D-shaped density (“pregnant lady” sign) as seen in a lateral chest film. Decubitus views are useful to determine if the collection is free flowing in the pleural space (stage I) or if it is loculated (stage II). Because it is often difficult to differentiate between lung consolidation and pleural fluid, computed tomographic (CT) scanning is used to ascertain the underlying pulmonary pathologic condition. It is also useful to stage the empyema as determined by the presence of loculations, thickness of the pleura, and presence or absence of a trapped lung. As reported by Stark and colleagues, visualization of thickened and separated pleural surfaces, compression of the parenchyma, and pleural thickening are specific CT signs of empyema.52 In 1991, Hanna and colleagues described the “split pleura sign,” which is indicative of the presence of pleural fluid between the thickened visceral and parietal pleurae.53
Ultrasonography may be used to document the presence of fluid or to distinguish between pleural fluid, pleural thickening, and parenchymal consolidation. It is also useful for guided needle aspiration of pleural fluid, especially when the position of the diaphragm cannot be documented with certainty on standard radiographs, and for entering loculated areas. As described by Moran54 and Orringer,55 an empyemagram can be done by injecting contrast material at the time of the initial thoracentesis and then obtaining posteroanterior and lateral chest films and decubitus views. Although this technique has seldom been used since the advent of CT, it may provide information about the extent of the empyema cavity and the presence or absence of loculations in the space. After the presence of pleural fluid has been confirmed, diagnostic thoracentesis should be done, and the aspirate should be sent for cytologic study, biochemical analysis, Gram stain, aerobic and anaerobic studies, and antibiotic sensitivity tests.
Orringer showed that the gross appearance and odor of pleural fluid are among the most significant items of information obtainable by thoracentesis.55 Thin fluid, even with positive bacteriologic findings, may respond to selective antibiotic therapy and therapeutic thoracentesis; thick pus requires formal surgical drainage. Anaerobic pus is usually foul; aerobic pus has no offensive odor. Several authors have shown that recovery of anaerobes requires careful technique. Varkey and colleagues noted that the variability in the reported incidence of anaerobic empyemas may be caused by differences in the methods of transportation and processing of the pleural fluid specimens.51 Pleural fluid should be sent for viral, tuberculosis, and fungal cultures in addition to the standard bacteriologic examinations.
The relevance of pleural fluid analysis in empyema diagnosis is controversial, especially with regard to its biochemistry. Several authors56–58 believe that pleural effusions with low fluid pH (<7.0), low glucose concentration (<50 mg/dL), and high LDH level (>1000 IU/L) should be drained because these parameters indicate a complicated effusion or impending empyema. These changes can be detected before organisms are found on Gram stain or culture, and they usually occur concomitantly. In uncomplicated effusions, the pH is greater than 7.3, the glucose level is higher than 60 mg/dL, and the LDH level is less than 1000 IU/L; these do not need to be drained. If the patient has free-flowing, nonpurulent fluid with borderline biochemical parameters, Sahn and Light recommend appropriate antibiotic therapy and repeated thoracentesis 12 hours later.59 If the pleural fluid values are stable or improving, continued antibiotic therapy is warranted, but if there is worsening of these values, chest tube or VATS drainage is generally necessary for resolution. In a series by Potts and colleagues, three categories of parapneumonic effusions were characterized.60 The pH was greater than 7.3 in all 10 benign effusions, and spontaneous resolution occurred in each case. All 10 empyemas and the four loculated effusions had pH levels that were less than 7.3.
Physiologically, these biochemical changes are explained by an increased leukocytic activity and acid production in the pleural fluid. Based on all these diagnostic parameters, Van Way III and colleagues proposed a method of regrouping patients with empyemas by diagnostic class.61 Patients with class I empyemas (n = 12) were treated with short-duration chest tubes, and there were no deaths. Patients with class II empyemas (n = 28) were treated with chest tubes, and there were two deaths (7%). There were 40 patients with class III empyemas, and most required some form of surgical intervention.
Despite the usefulness of all of these tests, the proper clinical staging of parapneumonic effusions remains difficult. How does one distinguish a simple inflammatory reaction (likely to respond to antibiotics and drainage) from early organization? How does one differentiate between the very acute stage in which pleural fluid is thin and the purulent stage in which fibrin is deposited over pleural surfaces? Values of pleural fluid chemistry, such as pH less than 7.2, correlate with loculated effusions but not necessarily with the presence of frank empyema.62 In experienced hands, CT and ultrasonography provide significant information by detecting loculations and thickness of the fibrinous deposits encasing the lung.63 Currently, VATS has been incorporated earlier to help determine stage and appropriate treatment. During the investigation of patients with empyema, it is also important to look for the causative process. Sullivan and colleagues, for example, showed that decayed teeth, retained food, or advanced periodontal disease were present in 17 of 24 patients with anaerobic empyemas of pulmonary origin.48 Bronchoscopy should be performed to rule out foreign bodies or endobronchial tumors, especially if the patient requires surgery.