Chapter 17 Emerging Technology in Surgery
Informatics, Robotics, and Electronics
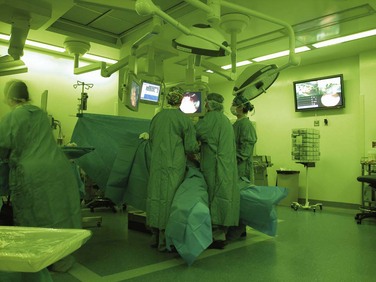
There has been a dramatic change in surgical care over the past 20 years with the introduction of digitization, miniaturization, improved optics, novel imaging techniques, and computerized information systems in the operating room (Fig. 17-1). Whereas surgery has traditionally required large incisions large enough to allow the surgeon to introduce his or her hands into the patient’s body and allow sufficient light to see the structures being operated on, innovations have stimulated a radical change in how surgical procedures are performed. Many surgical procedures have become image-guided. These can be done by manipulating instruments from outside the patient while directing them by looking at displays of direct images of the target tissues (e.g., endoscopic or laparoscopic surgery) or at indirect images of the region of interest (e.g., endovascular catheter-based treatments, energy-focused ablation of tumors). Image-guided surgery has enabled the use of very small incisions or punctures to introduce surgical instruments. In other cases, the surgical instruments can be passed to the target tissue through anatomic conduits (e.g., arteries, veins) or natural orifices (e.g., mouth, anus, vagina, urethra) without the need for any visible incision. Other innovations to minimize the damage of access to the patient include single-incision laparoscopic surgery and minilaparoscopy.
Minimally Invasive Surgery
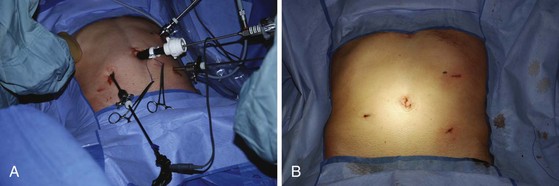
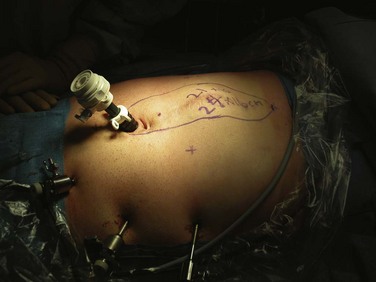
Accessing internal body cavities, such as the chest, abdomen, and pelvis, requires an incision. The size of the incision is determined by the need of the surgeon to see and manipulate the target tissues. If resection is required, the incision size must take into account the dimensions of the tissues to be removed. In some cases, thorough histologic examination of the removed tissue is not critical (e.g., splenectomy for idiopathic thrombocytopenic purpura [ITP], hysterectomy for fibroids) and the resected organ may be pulverized or morcellated to ease its removal through a small incision. In other cases, such as colectomy for cancer, it is important to examine the removed tissues accurately for staging and grading purposes and to ensure that the resection margins are free of disease. In the latter cases, the incision must be sufficient to avoid compromising the accuracy of the pathologic examination. The goal of minimal access surgery is to diminish the trauma of access without compromising the overall goal of the surgical procedure (Fig. 17-2).
Image-guided surgery greatly diminishes the postoperative pain and morbidity of wound infection and the longer term problems related to hernias and adhesions. However, the smaller incisions present some real challenges to the operating surgeon.1
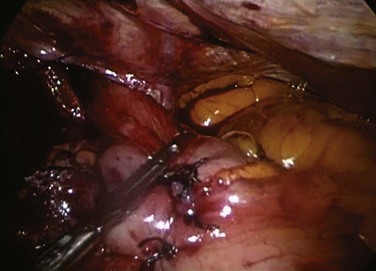
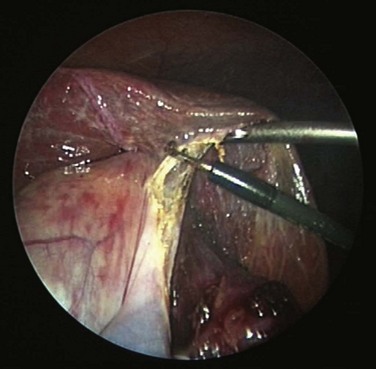
Laparoscopic surgery involves the placement of a small telescope into the body cavity. The telescope illuminates the target tissues and conveys a bright magnified, high-definition image to the surgeon through an attached or incorporated camera system. The view is startling in its clarity (Fig. 17-3). It eliminates shadows and affords all members of the operating team the identical view of the surgery. An important limitation of laparoscopic imaging is that it is generally monocular compared with the binocular view in open surgery, because traditional telescopes have a single-lens system. With a monocular optical system, the surgeon obtains a two-dimensional view of the body displayed on a video monitor. Other cues must be developed to appreciate the relative positions of the instruments and visualized tissues in three dimensions. This is a learned skill, and most surgeons are able to adjust to laparoscopic imaging with a little practice. The currently available robotic platform uses binocular imaging, providing the surgeon with a truly immersive three-dimensional view (Fig. 17-4).
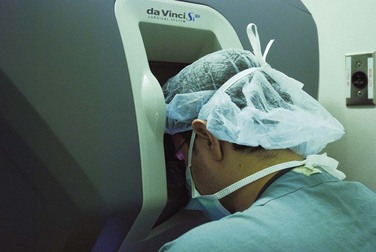
FIGURE 17-4 Surgeon is working in three-dimensional immersive visualization environment while doing robotic surgery.
Laparoscopic images give the surgeon a view of the surface of tissues. In open surgery, the surgeon can palpate and compress tissues to gain a sense of the presence of the pathology that lies deep to the surface. Because direct manual evaluation is not available during laparoscopy, the surgeon must adopt other methods to evaluate the tissues beneath the surface. Some of this information can be acquired before surgery by assessing the patient with cross-sectional imaging, such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). Digital CT and MR images can be displayed in the operating room using surface markers to help the surgeon consolidate these findings with the visual display of the tissue surface during surgery. An advantage of ultrasound (Fig. 17-5) is that it is easy to use intraoperatively and can be positioned to provide real-time information of the tissue being viewed through the laparoscope. A surgeon proficient in intraoperative ultrasound can incorporate the surface and cross-sectional information to evaluate the target tissues carefully.
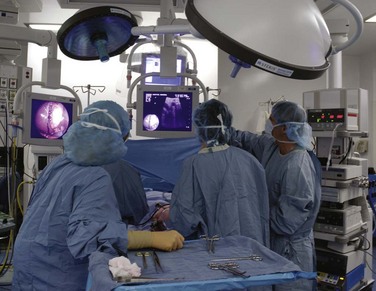
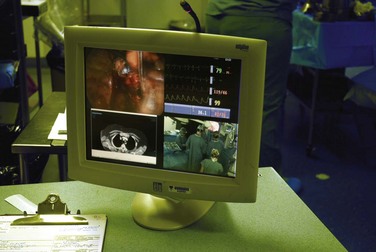
During minimally invasive surgery the surgeon’s eye is on the display monitor.2 One opportunity presented by laparoscopy is the display of multiple pieces of information on the imaging screen. Most data required by the surgical team are available digitally and can be routed to any display device. Because the anesthesiologist acquires important hemodynamic data on the patient, this information can be displayed on the surgeon’s monitor. When operating on an unstable patient, this provides valuable real-time data to the surgeon. Similarly, the surgeon can display real-time imaging information provided by intraoperative ultrasound, flexible endoscopy or fluoroscopy, or preoperatively acquired images (e.g., CT or MRI scans) simultaneously with the laparoscopic images using picture-in-picture, split screen, or quad split screen displays (Fig. 17-6). The surgeon can use telestration techniques (e.g., used to outline plays on TV sports broadcasts) to communicate with the assistant or point out findings to students (Fig. 17-7).
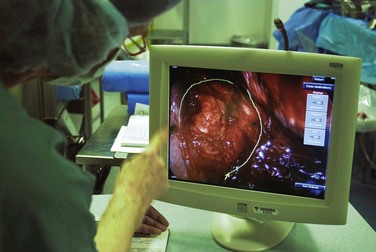
FIGURE 17-7 Telestration allows the surgeon to use the touch screen to annotate the image for documentation or teaching.
Videoconferencing is readily available, bringing consultant pathologists and radiologists right into the operating room environment. This provides useful context for their interpretation of findings. The surgeon can have access to a whole dashboard of information and select the relevant data for heads-up display during the procedure. The routing of digital information to display(s) can be carried out using voice controls or touch screens in the surgical field. The operating room images can also be accessed remotely in real time with appropriate security and privileges (Fig. 17-8).
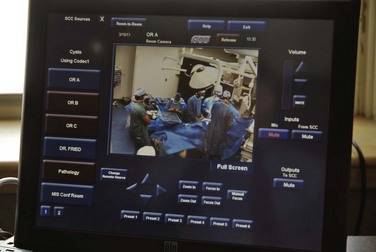
FIGURE 17-8 The touch interface in the surgeon’s office allows the image source to be selected and displayed remotely.
The operating room environment has been completely redesigned to provide an optimal setting for image-guided surgery.3 Ambient tinted room lighting provides the surgeon with a glare-free view of the display monitors while allowing others in the operating room (OR) sufficient illumination to move around the room and carry out their work safely. The surgical team has access to multiple monitors to display the surgical images and other digital information. It is not unusual to have six or seven monitors in an image-guided surgical suite. Each monitor can be moved into an ergonomically comfortable viewing position, and it has been shown that the monitor position has an impact on the precision and efficiency of the surgical procedure. Integrated ORs have been designed so that all devices, lighting, and image routing can be controlled from the surgical field or a control station. The surgeon can have access to multiple images simultaneously (e.g., laparoscope, flexible endoscope, ultrasound, fluoroscopy, preoperative CT and MRI images). By controlling the interface, any digital image or combination of images can be routed to any monitor. Any image can be recorded to document the surgical findings. The images or video clips can be annotated by verbal recordings or by textual description. This provides valuable documentation of the surgical findings for the medical record. Prompts can be embedded into the system so that a visual or auditory reminder can alert the surgical team when it is time for the next dose of antibiotics.
Despite the many advantages, image-guided surgery includes some unique challenges. When operating through a large incision, there are relatively few constraints on the range of motion of surgical instruments. If a surgeon wants to move the instrument tip upward, he or she can move the whole hand and instrument upward. In laparoscopy, the abdomen is insufflated with gas to create a working space. Generally, 5 to 6 liters of carbon dioxide is pumped into the abdominal cavity, separating structures and allowing the lens to focus on the target tissue from a suitable distance. To avoid loss of this working space, instruments must be passed through trocars or ports placed through the abdominal wall. These ports have gaskets that seal around the instruments, maintaining the positive pressure and working space. The design of these ports poses some limitations on instrument design. such as the geometry and curvature of the instrument shafts (Fig. 17-9). Because the handles of the instruments are outside the patient, the shafts are generally long. Interposing the laparoscopic instrument between the surgeon’s hands and the target tissue dampens tactile feedback. Surgeons rely on their determination of texture and compressibility to evaluate tissue characteristics and pathology. In laparoscopic surgery, the surgeon must learn how to interpret these characteristics through the instrument. In robotic laparoscopic surgery, the surgeon operates a device at a console outside the patient, which then controls instruments inside the patient. This results in complete loss of the sense of touch to evaluate tissues. Prototype instruments are being developed to display force feedback (tissue resistance) optically or by sound feedback. They have not yet been widely incorporated into clinical care.
Beyond Laparoscopic Surgery
One approach is to miniaturize the diameter of the surgical instruments and telescopes further (minilaparoscopy; Fig. 17-10). As camera light sensitivity and image quality improve, high-quality images can be obtained through progressively smaller laparoscopes. Whereas a 10- mm diameter telescope was once needed to provide sufficient light and image quality to perform surgery, current 5-mm telescopes can provide images that are hard to distinguish from those of 10-mm telescopes. Progressive reduction in their diameter allows the surgeon to move the telescope easily from port to port to provide different views of the surgical target and minimize the cosmetic and functional problems related to these incisions. In minilaparoscopy, surgeons can insert instruments as small as 2 mm into the body cavity through needle-sized incisions, leaving almost no scar. Other than the modification of instruments, there is no real difference in the conduct of the surgery, nor is further training required. The step from laparoscopy to minilaparoscopy is natural. Unfortunately, the reduced access size further constrains instrument design. Minilaparoscopy instruments are less robust and more limited in curvature than 5- or 10-mm instruments.4
Another approach has been to put the laparoscope and all instruments through a single port (usually placed at the depth of the umbilical dimple). This single incision is usually approximately 2 to 3 cm in length and can be placed in many patients so that the scar is almost invisible once healed. Placing all instruments through a single port creates further challenges because of instruments colliding with each other and creates a laparoscopic view that is parallel to the instruments, further impeding the surgeon’s depth perception. This has been addressed by designing deflectable tip laparoscopes and instruments with wristlike flexibility to help surgeons accommodate to these new challenges. Although there is clearly a learning curve for acquiring skills in single-incision laparoscopy, even for proficient laparoscopic surgeons, this learning curve can be overcome to a great extent by deliberate practice in the simulation environment.5
Natural orifice transluminal endoscopic surgery (NOTES) is a novel approach whereby access to the abdominal cavity is achieved without any incision in the abdominal wall.6 This is truly scarless surgery, conducted by accessing the abdominal cavity through a natural orifice (mouth, rectum, vagina). After placing a flexible or rigid endoscope through a natural orifice, an organ (stomach, colon, or vagina) is intentionally perforated and the endoscope is advanced into the peritoneal cavity. One way this is accomplished is by passing a flexible endoscope through the mouth into the stomach and through the stomach wall into the abdominal cavity. Other surgical instruments are advanced through or around the gastroscope and then out through this opening into the abdominal cavity. After the procedure is completed, the resected tissue is retrieved through the mouth and the gastrotomy is closed. As might be imagined, this technique is fraught with challenges. Very long instruments are required that need to be directed by manipulating the visualization platform (endoscope) using specially designed elevators at its distal end. This makes it difficult to move the instruments without moving the view. The operating platform is flexible and is unstable for the surgeon to work with. It also requires an iatrogenic perforation of a viscus to obtain access. Any failure of healing can result in peritonitis. Although access through the vagina is less risky than the stomach or colon, its potential use is limited to women, and the risk of postoperative dyspareunia remains to be determined. Currently, prospective trials are being conducted to compare NOTES with laparoscopic cholecystectomy. NOTES techniques may be more applicable to procedures other than cholecystectomy, such as those that already require an opening in the digestive tract.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree