Chapter 2
Embryology
Eric D. Endean, Bruce E. Maley
Based on a chapter in the seventh edition by Geoffrey D. Guttmann and Eric Endean
The fundamental purpose of the vascular system is to supply the organism with oxygen and nutrients and to remove metabolic waste products. During the first 3 weeks of gestation, simple diffusion is sufficient to support the embryo; however, by the fourth week, a functional cardiovascular system must be in place to maintain the rapidly developing embryo. The cardiovascular system in the embryo is one of the first and earliest systems to appear and begin to function. Isolated blood vessels and blood components form in the yolk sac by day 17, while just a day later isolated blood vessels begin to appear in the embryo proper. The heart begins to beat by day 22 and is pumping blood by day 24. In the past, the development of the vascular system has been viewed as no more than the growth of arteries, veins, and lymphatic vessels from a basic set of tubes. In reality, the appearance, growth, and remodeling of the vascular system are orchestrated by a series of signaling molecules, receptor molecules, and transcription factors. Failure of proper development of the vascular system can result in the appearance of variations or abnormalities that can have clinical ramifications. Knowledge of the development of the vascular system enables the vascular surgeon to recognize vascular anomalies and understand how they may have developed.
Formation of Embryonic Blood Vessels
Molecular Signaling of Vasculogenesis and Angiogenesis
The process by which the vascular system develops involves a series of steps. The first step is the modification of splanchnic mesodermal cells into angioblasts that form vesicular aggregates in the splanchnic mesoderm of the embryo and extraembryonic regions, including the yolk sac, connecting stalk, and the chorion.1 The angioblasts develop into flattened endothelial cells that form small vessel cords, which will, in turn, coalesce with one another to form vessels. This process, vasculogenesis, is under the control of signaling molecules secreted from underlying endoderm cells in each region. Vasculogenesis begins first in the yolk sac at day 17, where Indian hedgehog, bone morphogenic protein, and transforming growth factor β (TGF-β) induce the yolk sac’s mesoderm to form hemangioblastic aggregates. These cellular aggregates are composed of an inner core of hematopoietic stem cells and an outer rim of endothelial cells.2,3 The hematopoietic stem cells serve as the source of blood cells to the embryo until day 60, when the liver, spleen, thymus, and ultimately, the bone marrow become the source of the blood. Hemangioblastic aggregates also form in the connecting stalk and chorion at day 17. These aggregates coalesce to form the extraembryonic umbilical vessels that will act as the circulatory connection between the embryo and the maternal tissue. By day 18, the embryonic endoderm layer secretes bone morphogenic protein (BMP) and TGF-β to induce the splanchnic mesoderm to form vessels in a manner similar to what occurs in the yolk stalk. One of the first regions to develop a recognizable vascular system is at the cranial end of the embryo. Here, the splanchnic mesoderm forms a pair of endocardial tubes that are continuous with one another at their most rostral end as a horseshoe-shaped region called the cardiogenic area,1 the region that is the precursor of the heart. At the same time, additional embryonic splanchnic mesoderm also forms aggregates of hemangioblastic tissue that eventually will coalesce to form the paired dorsal aortas and will connect to the rostral end of the endocardial tubes by day 20. Extraembryonic vascular blood islands in the yolk sac and the connecting stalk eventually fuse with the developing embryonic vessels.1
Once the endothelial cells are established as vascular elements, they begin to sprout and bud, forming simple capillary networks. These capillary networks will be remodeled into arterial, capillary, and venous systems based on the surrounding environment. Additionally, some of the presumptive vessels will undergo vascular intussusception to remodel and generate additional vessels. Much of this growth is under the influence of vascular endothelial growth factor (VEGF), which activates a series of molecular and cellular events that ultimately lead to a stable vascular network.4 Angiogenic sprouting from existing vessels is facilitated by hypoxia, which upregulates a number of genes, including VEGF, angiopoietin-2, and nitric oxide synthase. Signaling molecules such as VEGF and angiopoietin-1 not only are proangiogenic factors, but also lead to endothelial specificity. VEGF expresses for a fenestrated endothelial layer within the capillaries of endocrine glands and the kidneys, whereas angiopoietin-1 expresses for tight junctions as seen in the capillaries of the brain (i.e., the blood-brain barrier) (Fig. 2-1A).4,5 As vessels mature, components of the extracellular matrix and the associated basement membrane stabilize the developing vasculature by acting as a source for a number of growth factors and proenzymes needed by the maturing vessels. The importance of the basement membrane in the maturation and maintenance of the vascular system is demonstrated by the number of vascular defects observed in animal models that have a deficiency of type IV collagen or laminins, key constituents of the basement membrane.5
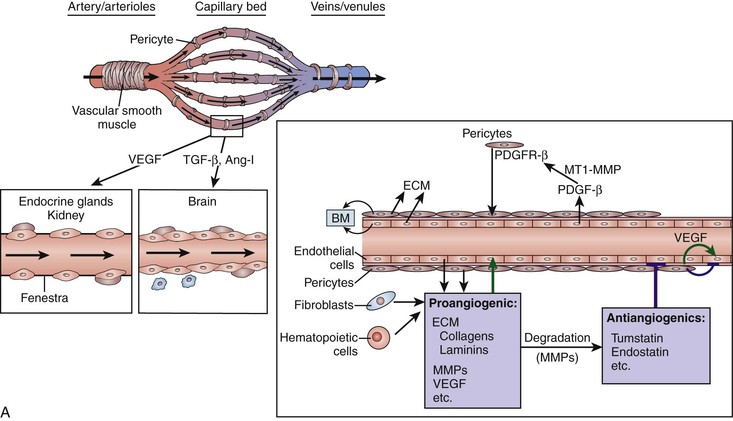
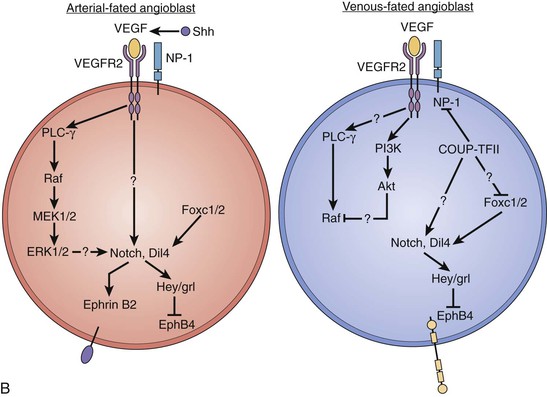
Figure 2-1 Initial development of vessels. A, Architectural features of the vasculature and their signaling molecules. B, Proposed molecular signaling pathways that determine the fate of angioblasts to become arterial or venous endothelial cells. Ang-I, Angiotensin I; BM, basement membrane; COUP-TFII, chicken ovalbumin upstream promotor transcription factor II; ECM, extracellular matrix; ERK, extracellular signal–regulated kinase; MEK, mitogen-activated protein kinase; MMPs, matrix metalloproteinases; MT1-MMP, membrane type 1 metalloproteinases; NP-1, neuropilin-1; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidyl-3′-kinase; PLC, phospholipase C; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Veins and arteries have been used to describe vessels whose blood flow direction is either to (veins) or from (arteries) the heart. Although there are morphologic differences in the walls of veins and arteries, it was not until recently that it was demonstrated that arteries and veins are also different with regard to their specific cell surface markers. Artery endothelial cells express ephrin B2 receptor, whereas vein endothelial cells express the EphB4 receptor. It is now known that angioblasts are fated to become either arterial or venous endothelial cells based on the attachment of VEGF either to the VEGF receptor 2 and neuropilin 1 (VEGFR2-NP-1) complex or simply to VEGFR2, respectively. The VEGFR2-NP-1 complex initiates a signaling cascade that leads to expression of the arterial cell surface marker ephrin B2. In angioblasts fated to be venous endothelial cells, VEGFR2 initiates a signaling cascade that leads to the inhibition of NP-1 by chicken ovalbumin upstream promotor transcription factor II (COUP-TFII) and finally to expression of the cell surface marker EphB4 (Fig. 2-1B).4,5 Lymphatic vessels develop through the process of angiogenesis from existing veins in specific sites of the developing vascular system. Budding of the lymphatic system from veins is under the direction of the gene, Prospero-related homeobox-1 (Prox-1), which is first expressed in a specific subpopulation of endothelial cells associated with the anterior cardinal veins. These cells ultimately bud, divide, and migrate from this venous structure to form lymph sacs.6 In embryos lacking the Prox-1 gene, the endothelial cells never acquire a lymphatic phenotype; rather, they keep a blood vascular phenotype. Other growth and transcription factors including VEGFR-3 and VEGFR-C have been shown to be critical for lymphatic endothelial sprouting and migration. The angiopoietin-2 gene is also critical for the correct patterning and integrity of lymphatic vessels; animals that lack angiopoietin-2 have lymphatics that are misshapen, leaky, and do not have the typical association of smooth muscle cells.7
Early Angiogenesis
As the major vascular structures including the heart, dorsal aortas, umbilical vessels, vitelline vessels, and cardinal veins are being formed by vasculogenesis, the embryo is already beginning to remodel this vascular system by the processes of angiogenesis and vascular intussusception. Other factors, especially related to the heart and dorsal aortas, have a profound effect on the definitive vascular system’s configuration. By day 20, the growth of the neural tube forces the cardiogenic area ventrally and caudally to its final position in the thoracic region, whereas lateral folding of the embryo causes the two enodcardial tubes to fuse along the midline, resulting in a single endocardial tube with a more cranially placed outflow and a more caudally located inflow region. As the presumptive heart lengthens, a series of swellings in the developing heart become visible. Beginning at the inflow end, these include the sinus venosus with its right and left horns, primitive atrium, primitive left ventricle, bulbus cordis (future right ventricle), and truncus arteriosus. The aortic sac, the rostral dilation of the truncus arteriosus, connects the developing heart to the dorsal aortas. As the developing heart moves into its ventral position, the attached dorsal aortas are forced into a dorsoventral bend that forms the first aortic arch between days 22 and 24. The first aortic arch is contained in the thickened mesoderm of the developing first pharyngeal arch surrounding the pharynx. Aortic arches 2 to 6 form from mesenchyme within their own pharyngeal arches in a rostral to caudal sequence between days 26 and 30. Lateral folding of the embryo also forces the paired dorsal aortas beginning at the fourth thoracic somite to fuse with one another as a common aorta; however, rostral to this level, the dorsal aortas remain as separate vessels. By the beginning of the fourth week, intersegmental vessels between each somite arise from the dorsal aortas. Each intersegmental vessel has a dorsal branch, a lateral branch, and a ventral branch that supply the individual somite regions.
Aortic Arch
Normal Arch Development
The vascular system in humans is symmetric, with the exception of the adult aortic arch. The right common carotid and right subclavian arteries arise from the brachiocephalic (innominate) artery, whereas the left common carotid and left subclavian arteries originate as separate vessels from the aortic arch. These differences are a consequence of the changes in the connections of the aortic arches, the dorsal aortas, and the aortic sac. Diagrammatic representation of these changes would suggest that the aortic arches are all present at the same time, but in reality, the first arches are already regressing while the others are still developing.
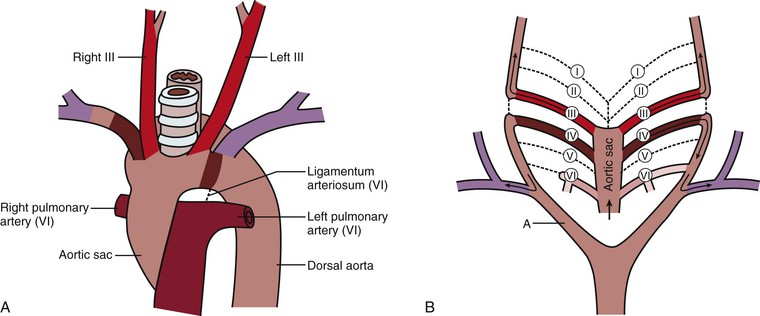
The first two aortic arches appear and regress quickly and contribute very little to adult structures, whereas the fifth aortic arch never develops in humans (Fig. 2-2A). The third aortic arches become the common carotid arteries and proximal segments of the internal carotid arteries. The distal segments of the internal carotid arteries are derived from the dorsal aorta between the first and third arches. The external carotid arteries sprout from the common carotid arteries. The dorsal aorta on each side of the embryo between the third and fourth arches disappears, thus directing blood through the third aortic arch system to the head and neck regions (Fig. 2-2B). The fourth aortic arches are asymmetrical with regard to their fate. The left aortic arch forms that part of the adult aortic arch between the left common carotid and left subclavian arteries, whereas the right aortic arch becomes the proximal segment of the right subclavian artery. The remainder of the right subclavian artery is derived from the right dorsal aorta and its right seventh intersegmental artery. The right dorsal aorta distal to the seventh intersegmental artery, but proximal to the fused common aorta, involutes (Fig. 2-2C). The right horn of the aortic sac, connected to the right third and fourth aortic arches, elongates to become the brachiocephalic (innominate) artery, and the left horn becomes the initial portion of the aortic arch. The proximal portions of both sixth arches become the right and left pulmonary arteries. The distal segment of the right sixth arch disappears, but the distal segment of the left sixth arch becomes the ductus arteriosus during fetal life and atrophies after birth to become the ligamentum arteriosum. The ductus arteriosus in the fetus acts as a shunt between the left pulmonary artery and the aorta to move blood away from the immature fetal lungs to the aorta (Fig. 2-2D).
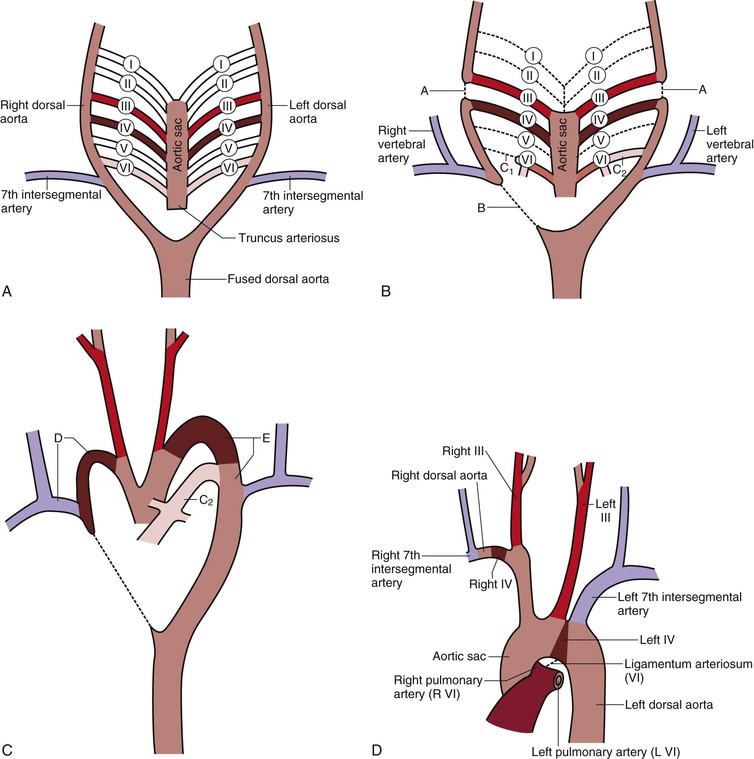
Figure 2-2 A, The six primitive aortic arches. The first, second, and fifth arches disappear. The paired dorsal aortas fuse at the level of the seventh cervical vertebra to become the thoracic and abdominal aorta. B, Differentiation of the aortic arches. The dorsal aorta between the third and fourth arches (A) involutes so that blood entering the third arch perfuses the head only. The third arch and its branches become the carotid system. The right dorsal aorta distal to the right seventh intersegmental artery (B) involutes so that blood entering the right fourth arch perfuses the right upper limb. This system becomes the right subclavian artery. The distal part of the right sixth arch (C1) involutes, but the distal part of the left sixth arch (C2) persists and becomes the ductus arteriosus. C, Development of the aortic arches. The right fourth arch, the dorsal aorta distal to the right fourth arch, and the right seventh intersegmental artery become the right subclavian artery (D). The left fourth arch and the left dorsal aorta distal to it become part of the aortic arch (E). The left seventh intersegmental artery becomes the left subclavian artery. Pulmonary arteries form from the sixth arch with the ductus arteriosus (C2) present. D, Segments of the aortic arches that produce the adult aortic arch. The patent ductus arteriosus becomes the ligamentum arteriosum.
Aortic Arch Anomalies
In light of the complexity of events that must occur for normal development of the aortic arch and its branches, anomalies do occur.8–14 Anomalies result when segments of the primitive aortic arch that should disappear persist or other parts that should persist disappear. Variations in the development of vessels as they arise from the aortic arch are relatively common. The most common developmental pattern (“normal”) is described in the preceding sections and illustrated in Figure 2-2D. This pattern of development occurs in 65% of the population. In 22% of the population, the left common carotid artery originates from the brachiocephalic trunk rather than from the aortic arch in what is termed a “bovine arch.” In this case, the brachiocephalic trunk gives rise to the right subclavian, right common carotid, and left common carotid arteries, whereas the left subclavian artery originates from the aortic arch as normally expected. This variant accounts for 73% of all arch anomalies. Many other variations, each occurring in less than 3% of the population, have been described. Some of these anomalies include a very shortened brachiocephalic trunk that bifurcates immediately into the right subclavian and right common carotid arteries, with the left common carotid arising from the aortic arch at the base of the brachiocephalic trunk and a normal origin for the left subclavian artery from the aortic arch. The left vertebral artery can originate directly from the aortic arch between the left common carotid and left subclavian arteries. A left brachiocephalic trunk may be present, which bifurcates into left subclavian and left common carotid arteries.8
Patent Ductus Arteriosus.
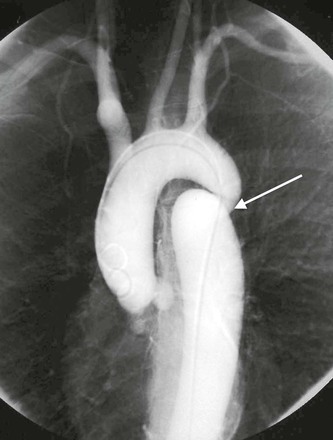
The ductus arteriosus develops from the distal portion of the left sixth aortic arch and is responsible for shunting blood from the developing lungs to the systemic circulation. A patent ductus arteriosus is the most common vascular anomaly. At birth, the ductus normally constricts, which is probably due to the response of oxygen-sensitive smooth muscle cells in its wall to exposure of the high content of oxygen in the blood. By the age of 1 month, the ductus normally obliterates to become the ligamentum arteriosum. If the ductus arteriosus does not constrict and remains patent, blood is shunted from the high-pressure thoracic aorta to the low-pressure pulmonary system, eventually resulting in significant pulmonary hypertension (Fig. 2-3).
Coarctation of the Aorta.
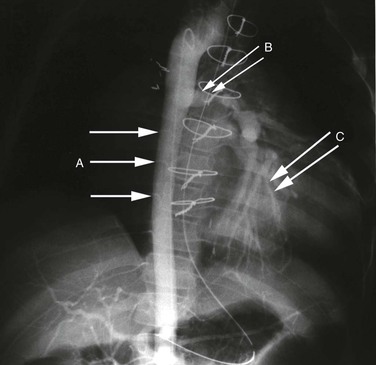
Aortic coarctation usually occurs at the level of the ligamentum arteriosum, but can be seen at any level of the aorta. The most common location is postductal, just distal to the ligamentum arteriosum; the preductal type occurs immediately proximal to the ligamentum. The etiologic mechanism of coarctation remains unclear, but it is hypothesized that its development is similar to the process that results in normal, physiologic obliteration of the ductus arteriosus. It is thought that oxygen-sensitive muscle tissue from the ductus arteriosus is incorporated into the wall of the aorta. This smooth muscle, which constricts when exposed to high oxygen tension, results in narrowing of the aorta at this level. Eventually, chronic changes develop, and the constriction becomes permanent. Relative ischemia of the body below the constriction stimulates the development of collateral vessels. Radiographically, notching on the inferior aspect of the third to eighth ribs may be seen as a consequence of the increased collateral blood flow in the intercostal arteries (Figs. 2-4 and 2-5).6,14,15
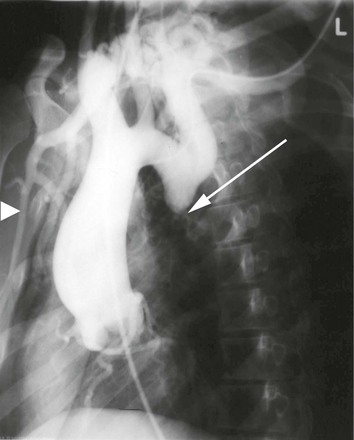
Figure 2-4 Coarctation of the aorta is identified by the arrow. Note the enlarged great vessels. The right internal mammary (arrowhead), a major collateral vessel to the lower portion of the body, is very visible and enlarged.
Double Aortic Arch.
The right dorsal aorta distal to the right seventh intersegmental artery may fail to involute, and the result is a double aortic arch. This segment passes posterior to the esophagus and joins the left aortic arch, which passes anterior to the trachea. As a result, a vascular ring forms around the esophagus and trachea. Symptoms may develop from compression of the esophagus and/or trachea (Fig. 2-6).
Right Aortic Arch.
Involution of the left dorsal aorta distal to the left seventh segmental artery with persistence of the right dorsal aorta (opposite the normal sequence) creates a right aortic arch (Fig. 2-7). The ligamentum arteriosum arises from the distal right sixth arch instead of the distal left sixth arch, but still connects to the aorta. If the arch passes to the left side and posterior to the esophagus, a vascular ring is formed with the ligamentum arteriosum and is known as a right aortic arch with an aberrant left subclavian artery or a retroesophageal component. If the right aortic arch passes anterior to the esophagus and trachea, a vascular ring is not formed, and it is considered to be a right aortic arch with mirror image branching. The former anomaly may initially be a double aortic arch in which the left dorsal aorta later regresses (see Fig. 2-3). The latter is a mirror image of normal anatomy that is associated with a higher incidence of heart malformations in which almost all infants are cyanotic. An example of a heart malformation associated with mirror image branching of the aortic arch is tetralogy of Fallot.14
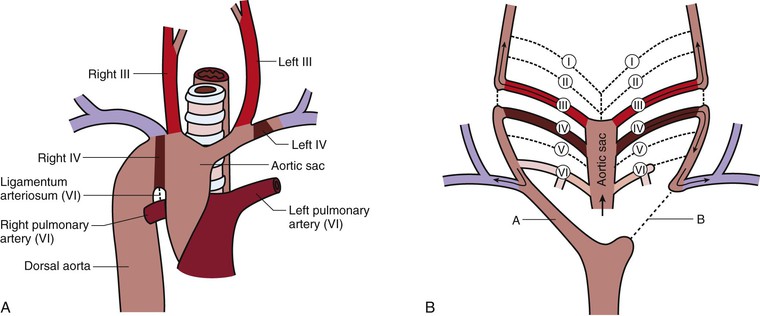
Figure 2-7 Right aortic arch. A, One of two types in which the right arch passes anterior to the trachea and esophagus so that no vascular ring is formed. If the right arch passes posterior to the trachea and esophagus, the ligamentum arteriosum and the right arch form a vascular ring. B, The right dorsal aorta distal to the right seventh intersegmental artery persists (A), but the left dorsal aorta distal to the left seventh intersegmental artery involutes (B), the opposite of the normal occurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree