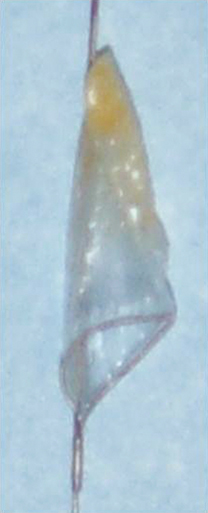
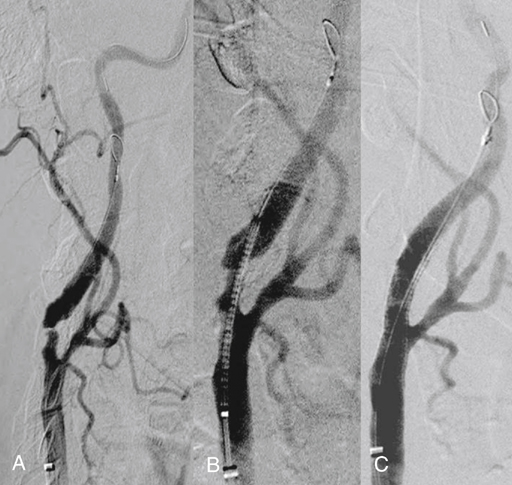
Microporous filters are designed to catch embolic debris that is liberated during CAS before it reaches the cerebral circulation. A delivery sheath constrains the filter until it is positioned in a nondiseased segment of the internal carotid artery distal to the area to be stented. Once the filter is deployed, antegrade cerebral perfusion is maintained through the filter throughout the procedure. Embolic material dislodged during balloon angioplasty becomes trapped within the filter and is removed when the filter is reconstrained at the completion of the procedure (Figure 1). Pore sizes for filters range from 100 to 165 μm, allowing capture of particulate debris larger than the pore size yet preserving blood flow through the filter. If a fixed-wire filter system is chosen, the wire with the filter inside the delivery system is advanced past the lesion under fluoroscopic visualization into a straight segment of internal carotid artery a few centimeters distal to the proposed level of stent placement. Deployment of the filter in an arterial segment in which the walls are parallel allows optimal apposition of the filter to the luminal surface of the internal carotid artery. The delivery system is removed, resulting in deployment of the self-expanding filter. Angiography is performed to confirm proper placement, adequate wall apposition, and preservation of antegrade cerebral blood flow (Figure 2).
Embolic Protection Devices to Prevent Stroke during Percutaneous Angioplasty and Stenting
Device Characteristics
Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine