Whether an additional clopidogrel load in patients receiving chronic clopidogrel therapy and undergoing percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS) is associated with clinical benefit has not been well characterized. The aim of the present study was to evaluate, in a randomized protocol, the safety and effectiveness of clopidogrel reload for patients with ACS undergoing PCI in the background of chronic clopidogrel therapy. A total of 242 patients with non–ST-segment elevation ACS with >10 days of clopidogrel therapy randomly received a 600-mg loading dose of clopidogrel 4 to 8 hours before PCI (n = 122) or placebo (n = 120). The primary end point was the 30-day incidence of major adverse cardiac events (death, myocardial infarction, target vessel revascularization). The primary end point occurred in 4.1% of patients in the reload arm versus 14.1% in the placebo arm (odds ratio 0.26, 95% confidence interval 0.10 to 0.73, p = 0.013). This benefit in the reload arm was mainly from the prevention of periprocedural myocardial infarction (4.1% vs 13%, p = 0.02) and was paralleled by lower periprocedural platelet reactivity. The aggregometry data were consistent with the clinical outcome. No difference was found in the bleeding outcomes between the 2 groups. In conclusion, the results from the Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty (ARMYDA-8 RELOAD-ACS) trial have shown a significant clinical benefit from reloading patients with ACS receiving chronic clopidogrel therapy before PCI. These data might be relevant in clinical practice, given the large number of patients with ACS who are still currently treated with clopidogrel during PCI.
The prevalence of patients undergoing percutaneous coronary intervention (PCI) receiving chronic clopidogrel therapy is relatively high, owing to previous drug-eluting stent implantation, acute coronary syndrome (ACS) during the preceding year, or staged PCI procedures. An interindividual variability in the clopidogrel response has been largely demonstrated, and patients with low drug responsiveness have a poorer outcome after PCI. Previous studies have shown that an additional 600-mg clopidogrel loading dose in patients receiving chronic therapy is associated with additional inhibition of residual platelet reactivity and a reduction of nonresponders. However, whether this pharmacodynamic benefit translates into improvement in the clinical outcomes has not been definitely characterized. A clinical benefit from an additional loading dose with 600 mg clopidogrel in patients with ACS undergoing PCI while receiving chronic clopidogrel therapy was demonstrated in the prespecified subgroup analysis of the randomized Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty (ARMYDA-4 RELOAD) trial, but this was not observed in a more recent, nonrandomized, retrospective investigation. Thus, given those discrepancies, we designed a specific, prospective, randomized trial to evaluate the effectiveness and safety of a strategy of a 600-mg clopidogrel reload dose in patients with ACS undergoing PCI while receiving chronic clopidogrel therapy. The Clinicaltrials.gov number was 2011-005449-11 .
Methods
The ARMYDA-8 RELOAD-ACS trial was a multicenter, unfunded, randomized, double-blind, clinical trial performed at 4 institutions (Campus Bio-Medico University of Rome, Rome, Italy; Vito Fazzi Hospital, Lecce, Italy; San Filippo Neri Hospital, Rome, Italy; and OLV Hospital, Aalst, Belgium).
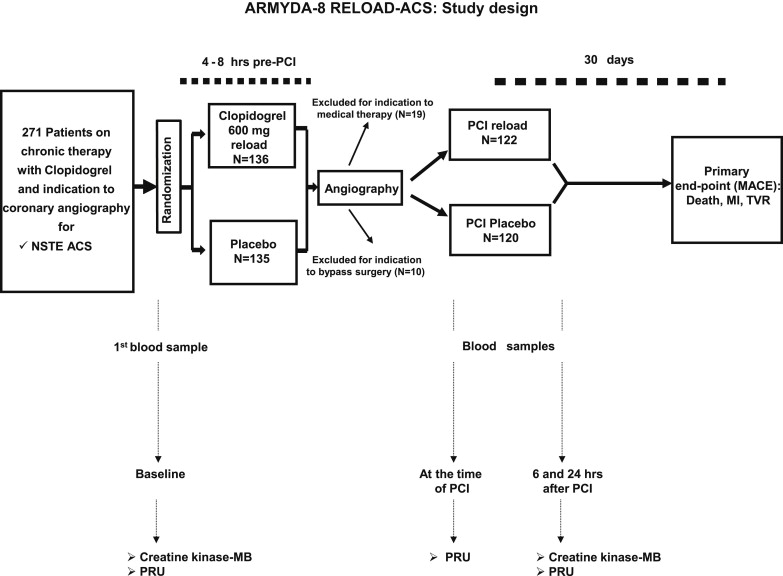
By protocol, patients were eligible if they were receiving chronic (>10 days) therapy with clopidogrel (75 mg/day) and had non–ST-segment elevation ACS. Non–ST-segment elevation ACS was defined as symptoms of coronary ischemia <24 hours before hospital admission and ≥1 of the following findings: troponin or creatine kinase-MB (CK-MB) values greater than the upper limit of normal, or new ST-segment depression >0.1 mV, or transient ST-segment elevation (<30 minutes) >0.1 mV in ≥2 contiguous leads. Excluded were the patients undergoing primary PCI for ST-segment elevation acute myocardial infarction, with a platelet count <70 × 10 9 /L, coronary bypass grafting in the previous 3 months, chronic warfarin therapy, bleeding diathesis, or major bleeding for <4 weeks. The design of the study is illustrated in Figure 1 . A total of 271 patients fulfilling the enrollment criteria were randomized 4 to 8 hours before diagnostic angiography to receive an additional 600-mg clopidogrel loading dose (n = 136) or placebo (n = 135). The eligible patients were assigned to the allocation arm using an electronic spreadsheet indicating the group assignment by random number. Randomization blocks were created and distributed to all centers; in each center, the investigators involved in the process of the randomization assignment were not involved in performing PCI or the follow-up evaluations. After coronary angiography, 29 patients (14 in the reload arm and 15 in the placebo arm) without an indication for PCI were excluded from the present study (19 were treated medically and 10 underwent bypass surgery). Thus, a total of 242 patients receiving “ad hoc” PCI immediately after coronary angiography were enrolled and represent the study population. Of these 242 patients, 122 were randomized to the 600-mg clopidogrel reloading dose and 120 to placebo. The physicians performing PCI were not aware of the randomization allocation.
All interventions were performed using a standard technique. Glycoprotein IIb/IIIa inhibitors were used at the operator’s discretion in both arms. All patients were taking aspirin at PCI. After the procedure, they received aspirin (100 mg/day) indefinitely. Clopidogrel was continued (75 mg/day) for 12 months, irrespective of the randomization assignment.
Blood samples were drawn before intervention and 6 and 24 hours after PCI to measure the CK-MB and troponin I (mass) values. Additional determinations were performed, if clinically indicated. The measurements of CK-MB were obtained using the Access 2 Immunochemiluminometric assay (Beckman Coulter, Fullerton, California). The normal limits were ≤3.6 ng/ml for CK-MB and ≤0.06 ng/ml for troponin I. Patients treated at Campus Bio-Medico University received platelet reactivity evaluation at baseline (4 to 8 hours before intervention), in the catheterization laboratory immediately before PCI and 6 and 24 hours after PCI using the VerifyNow P2Y12 assay (Accumetrics, San Diego, California). VerifyNow P2Y12 is a rapid, cartridge-based assay specifically measuring the direct effects of clopidogrel on the platelet P2Y12 receptor. The measurement results are expressed as P2Y12 reaction units (PRU); the lower the PRU value, the greater the degree of P2Y12 receptor inhibition by clopidogrel, and vice versa. Patients treated with glycoprotein IIb/IIIa inhibitors were excluded from the VerifyNow analysis, because these drugs interfere with the PRU measurements.
Clinical follow-up data were obtained at 30 days by office visits for all study patients. The physicians evaluating the patients during these follow-up visits were not aware of the randomization assignment. Each patient gave informed consent for participation in the study. The institutional review board of the Campus Bio-Medico University approved the study. The trial was not supported by any external source of funding.
The primary end point of the ARMYDA-8 RELOAD-ACS trial was the 30-day incidence of major adverse cardiac events (MACE; death, myocardial infarction, target vessel revascularization). Periprocedural myocardial infarction was defined according to the pre-PCI clinical presentation. For patients with normal baseline CK-MB levels (i.e., undergoing angioplasty for unstable angina), it was defined as postintervention increases in CK-MB >3 × 99th percentile of the upper limit of normal. In patients with non–ST-segment elevation myocardial infarction (i.e., elevated baseline CK-MB levels), a subsequent elevation of ≥50% of the baseline CK-MB value was applied to detect periprocedural myocardial infarction. Target vessel revascularization included bypass surgery and repeat PCI of the target vessels.
The secondary end points were as follows: (1) the occurrence of vascular or bleeding complications, including major bleeding (intracranial bleeding or clinically overt bleeding associated with a decrease in hemoglobin >5 g/dl, according to the Thrombolysis In Myocardial infarction criteria ), minor bleeding (clinically overt hemorrhage associated with a decrease in hemoglobin of ≤5 g/dl), and entry-site complications (hematoma >10 cm, pseudoaneurysm or arteriovenous fistula), and (2) the evaluation of periprocedural platelet reactivity at different points in the 2 treatment arms using the VerifyNow assay.
In the ARMYDA-4 RELOAD study, subgroup analysis using prespecified clinical subsets of patients with ACS, showed a MACE incidence of 6.4% in the reload arm versus 16.3% in the placebo arm (odds ratio [OR] 0.34, multivariate analysis). For the sample size calculation of the ARMYDA-8 RELOAD-ACS, we hypothesized a similar 16% occurrence of MACE in the placebo group and a similar 66% risk reduction in the clopidogrel reload arm. Thus, a study population of ≥240 patients would be needed to detect such a reduction with an α of 0.05 (2 tailed) and a β of 0.08.
Categorical variables are expressed as percentages and continuous variables as the mean ± SD, unless otherwise specified. Proportions were compared using Fisher’s exact test when the expected frequency was <5, otherwise the chi-square test (Yates’ corrected) was applied. Continuous variables between the 2 arms were compared using the t test for normally distributed values (as assessed by the Kolmogorov-Smirnov test), otherwise, the Mann-Whitney U test was used. ORs and 95% confidence intervals assessing the risk of the primary end point according to potential confounding variables were assessed by logistic regression analysis. All variables listed in Tables 1 and 2 were evaluated first in a univariate model, and, for those with p <0.15, in a multivariate logistic regression analysis. All calculations were performed using the Statistical Package for Social Sciences, version 12.0, and p <0.05 (2 tailed) was considered significant.
| Variable | Clopidogrel Reload (n = 122) | Placebo (n = 120) | p Value |
|---|---|---|---|
| Age (yrs) | 66 ± 11 | 66 ± 12 | 1 |
| Men | 94 (77) | 84 (70) | 0.2 |
| Diabetes mellitus | 33 (27) | 37 (31) | 0.6 |
| Systemic hypertension ∗ | 86 (70) | 95 (79) | 0.1 |
| Hypercholesterolemia † | 82 (67) | 76 (63) | 0.6 |
| Current smoker | 31 (25) | 32 (27) | 0.9 |
| BMI (kg/m 2 ) | 24.1 ± 4.2 | 24.8 ± 4.3 | 0.2 |
| Previous MI | 23 (19) | 36 (30) | 0.052 |
| Previous PCI | 29 (24) | 44 (37) | 0.036 |
| Previous coronary bypass | 6 (5) | 9 (7) | 0.4 |
| Unstable angina pectoris | 44 (36) | 48 (40) | 0.6 |
| NSTEMI | 78 (64) | 72 (60) | 0.6 |
| LVEF (%) | 57 ± 9 | 56 ± 9 | 0.4 |
| Multivessel coronary artery disease | 53 (43) | 55 (46) | 0.8 |
| Blood creatinine (mg/dl) | 0.94 ± 0.23 | 0.99 ± 0.3 | 0.15 |
| Therapy | |||
| Aspirin | 122 (100) | 120 (100) | 1 |
| Statins | 110 (90) | 102 (85) | 0.2 |
| PPIs | 67 (55) | 77 (64) | 0.18 |
| β Blockers | 59 (48) | 63 (52) | 0.5 |
| ACE inhibitors | 79 (65) | 69 (57) | 0.3 |
∗ Arterial pressure >160/90 mmHg.
| Variable | Clopidogrel Reload (n = 122) | Placebo (n = 120) | p Value |
|---|---|---|---|
| Femoral access | 122 (100) | 120 (100) | 1 |
| Coronary vessel treated | 131 (100) | 144 (100) | – |
| Left main | 1 (0.5) | 2 (1.5) | 1 |
| Left descending artery | 56 (43) | 66 (46) | 0.6 |
| Left circumflex | 34 (26) | 35 (24) | 0.8 |
| Right | 39 (30) | 38 (26) | 0.6 |
| Saphenous vein graft | 1 (0.5) | 2 (1.5) | 1 |
| Left internal mammary graft | 0 | 1 (1) | 1 |
| Restenotic lesion | 10 (8) | 13 (11) | 0.5 |
| Lesion type B2/C | 82 (67) | 76 (63) | 0.6 |
| Chronic total occlusion (>3 mo) | 3 (2) | 3 (2) | 1 |
| Multivessel coronary intervention | 17 (14) | 22 (18) | 0.4 |
| Type of intervention | |||
| Balloon only | 6 (5) | 6 (5) | 1 |
| Stent | 116 (95) | 114 (95) | 1 |
| Bifurcation with kissing balloon | 7 (6) | 5 (4) | 0.8 |
| Stents per patient (n) | 1.3 ± 0.8 | 1.3 ± 0.9 | 1 |
| Stent diameter (mm) | 2.9 ± 0.9 | 2.9 ± 0.9 | 1 |
| Total stent length (mm) | 16.5 ± 6 | 16 ± 6 | 0.5 |
| Use of drug-eluting stent | 57 (47) | 52 (43) | 0.6 |
| Direct stenting | 49 (40) | 42 (35) | 0.4 |
| Predilation (n) | 1.3 ± 1.7 | 1.5 ± 2 | 0.4 |
| Stent deployment pressure (atm) | 12 ± 4 | 12 ± 4 | 1 |
| Duration of stent deployment (s) | 21 ± 8 | 21 ± 9 | 1 |
| Total myocardial ischemia >120 s | 23 (19) | 25 (21) | 0.7 |
| Use of postdilation | 3 (2) | 4 (3) | 0.7 |
| Antithrombin regimen during intervention | |||
| Bivalirudin | 13 (11) | 7 (6) | 0.2 |
| Unfractionated heparin | 109 (89) | 113 (94) | 0.3 |
| Use of GPIIb/IIIa inhibitors (provisional) | 14 (11) | 19 (16) | 0.3 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


