Ventricular septal myectomy in patients with obstructive hypertrophic cardiomyopathy (HC) has been shown to reduce left ventricular (LV) outflow tract (LVOT) gradient and improve symptoms, although little data exist regarding changes in left atrial (LA) volume and LV diastolic function after myectomy. We investigated changes in LA size and LV diastolic function in patients with HC after septal myectomy from 2004 to 2011. We studied 25 patients (age 49.2 ± 13.1 years, 48% women) followed for a mean of 527 days after surgery who had serial echocardiography at baseline and at most recent follow-up, at least 6 months after myectomy. In addition to myectomy, 3 patients (12%) underwent Maze surgery and 13 (52%) underwent mitral valve surgery, of whom 5 had a mitral valve replacement or mitral annuloplasty. Patients with mitral valve replacement or mitral annuloplasty were excluded from LV diastolic function analysis. LA volume index decreased (from 47.2 ± 17.6 to 35.9 ± 17.0 ml/m 2 , p = 0.001) and LV diastolic function improved with an increase in lateral e′ velocity (from 7.3 ± 2.9 to 9.8 ± 3.1 cm/sec, p = 0.01) and a decrease in E/e′ (from 14.8 ± 6.3 to 11.7 ± 5.5, p = 0.051). Ventricular septal thickness and LVOT gradient decreased, and symptoms of dyspnea and heart failure improved, with reduction in the New York Heart Association functional class III/IV symptoms from 21 (84%) to 1 (4%). In conclusion, relief of LVOT obstruction in HC by septal myectomy results in improved LV diastolic function and reduction in LA volume with improved symptoms.
Hypertrophic cardiomyopathy (HC) is a common genetic cardiovascular disease that causes symptomatic left ventricular (LV) outflow tract (LVOT) obstruction in a proportion of patients resulting in symptoms of heart failure. Atrial fibrillation (AF) with or without thromboembolic complications or sudden cardiac death occurs in about 13% of patients. Septal myectomy is an established therapy for patients with obstructive HC with symptoms refractory to maximal medical therapy and results in improved LVOT obstruction, decreased mitral regurgitation, and symptomatic improvement. Left atrial (LA) dilation has been shown to be associated with an increased incidence of AF and stroke, and in patients with HC, it has also been shown to be a marker for decreased exercise tolerance and increased incidence of morbidity and mortality related to heart failure and stroke. Although reduction in LVOT gradient and improvement in symptoms are well documented, little data exist regarding changes in LA volume and LV diastolic parameters after septal myectomy in HC. We sought to further explore whether LV diastolic function and LA size improve after septal myectomy with or without mitral valve repair and whether there is a decreased incidence of AF after this procedure.
Methods
All patients were identified from the Bluhm Cardiovascular Institute’s Clinical Trials Unit Cardiovascular Research Database approved by the Institutional Review Board at Northwestern University (IRB #STU00012288). Preoperative, operative, and postoperative data were obtained from the database and medical record review, and data were de-identified before analysis. Patients who did not opt to participate in the research database are not included in this study. Patients aged ≥18 years who underwent septal myectomy for refractory symptoms on maximally tolerated medical therapy, with or without concomitant mitral valve or Maze surgery, at our institution from June 1, 2004, to December 31, 2011, were eligible for inclusion in the study. Analysis of preoperative and postoperative echocardiograms was performed. The postoperative echocardiogram selected for analysis was the most recent echocardiogram after the first 6 months after surgery. Patients were excluded if preoperative and postoperative echocardiograms were not available for review. Obstructive HC was defined by the presence of symptoms with a peak LVOT gradient of ≥30 mm Hg at rest or ≥50 mm Hg with provocation or at rest. Patients were noted to have AF if it was documented by electrocardiogram, ambulatory monitoring, or telemetry, only if it occurred after 30 days from surgery.
Using the recommendations from the American Society of Echocardiography, 2-dimensional and Doppler echocardiographic measurements were performed. In the apical 4-chamber view, pulse-wave Doppler was used at the tips of the mitral valve to assess transmitral valve filling velocities including peak early (E) and late (A) velocities, E/A ratio, and deceleration time. The peak early diastolic annular velocity (e′) was obtained using tissue Doppler at the lateral mitral annulus. Patients were excluded from the analysis of diastolic parameters if they underwent mitral valve ring annuloplasty repair or mitral valve replacement (MVR) as tissue Doppler and mitral inflow parameters are unreliable in this setting. LA volume was measured using the biplane area-length method, and LA area was measured in the apical 2- and 4-chamber views at end-systole before the opening of the mitral valve excluding the atrial appendage and pulmonary veins. LA length was measured in both views, and the shortest length was used to calculate LA volume using the following equation: 0.85 × 2-chamber area × 4-chamber area/LA length. Right atrial pressure was estimated to be 3 mm Hg if the inferior vena cava was <2.1 cm and collapsed >50% during inspiration and 15 mm Hg if the inferior vena cava was >2.1 cm and collapsed <50% during inspiration. Intermediate inferior vena cava measurements and collapse during inspiration were estimated to be 8 mm Hg. Pulmonary artery systolic pressure was estimated using the simplified Bernoulli equation: (4 × [tricuspid regurgitation velocity squared]) + right atrial pressure. LVOT gradient was measured using continuous-wave Doppler in either the apical 4-chamber or apical 3-chamber view to determine the peak gradient at rest.
Paired t tests were used to compare preoperative and postoperative continuous data. Fisher’s exact test was used to compare categorical data preoperatively and postoperatively. Statistical significance was declared at a p value <0.05.
Results
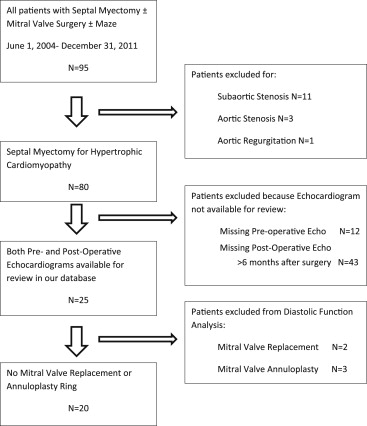
A total of 95 patients who underwent septal myectomy with or without mitral valve surgery or Maze were identified. Of these patients, 15 were excluded because they did not have underlying HC (11 with discrete subaortic stenosis, 3 with aortic stenosis, and 1 with aortic regurgitation) and 55 were excluded because they did not have 1 of the 2 echocardiograms available for review at our institution (12 missing a preoperative echocardiogram, 43 missing an echocardiogram >6 months after myectomy; Figure 1 ). Twenty-five patients (age 49.2 ± 13.1 years, 48% female) who met inclusion criteria were identified. Patients were followed for an average of 527 days after surgery. Of the 25 patients, 5 also underwent mitral valve surgery (2 with MVR and 3 with mitral valve repair with annuloplasty ring) precluding postoperative assessment of diastolic parameters including tissue Doppler velocities. Two of the patients who had a mitral valve annuloplasty ring placed also had an Alfieri stitch repair, and an additional 8 patients had an Alfieri stitch mitral valve repair in the absence of annuloplasty ring placement at the time of septal myectomy. Baseline chamber dimensions, LV ejection fraction, and LVOT gradients did not differ between patients who did not undergo any concomitant mitral valve surgery compared with those who did. Blood pressure and heart rates were well controlled before and after surgery.
Before septal myectomy, 23 patients (92%) had dyspnea and 21 (84%) were in New York Heart Association (NYHA) functional class III/IV. After surgery, symptoms significantly improved with only 3 patients (12%) with residual dyspnea and 1 (4%) remaining in NYHA class III/IV (p <0.001 for both). There was also a decrease in the proportion of patients with moderate to severe mitral regurgitation, which occurred in 10 patients (40%) before surgery and 3 patients (12%) after surgery ( Table 1 ).
| Variable | Pre-Operative (n = 25) |
|---|---|
| Age at surgery (years) | 49 ± 13 |
| Women | 12 (48%) |
| Mitral valve surgery | 13 (52%) |
| Alfieri repair | 10 (40%) |
| Annuloplasty | 3 (12%) |
| Replacement | 2 (8%) |
| Maze surgery | 3 (12%) |
| Coronary bypass | 0 |
| Time to post-operative echo from surgery (days) | 527 |
| Pre-Operative and Follow-Up Comparison | |||
|---|---|---|---|
| Pre-Operative (n = 25) | Follow-Up (n = 25) | p-Value | |
| Systolic blood pressure (mm Hg) | 114 ± 23 | 120 ± 18 | 0.08 |
| Diastolic blood pressure (mm Hg) | 70 ± 14 | 72 ± 10 | 0.41 |
| Heart rate (beats/minute) | 72 ± 11 | 73 ± 13 | 0.63 |
| LVOT gradient >30 mm Hg at rest | 14 (56%) | 4 (16%) | 0.01 |
| Atrial fibrillation | 4 (16%) | 3 (12%) | NS |
| Dyspnea | 23 (92%) | 3 (12%) | <0.001 |
| NYHA functional class III/IV | 21 (84%) | 1 (4%) | <0.001 |
| Moderate/severe mitral regurgitation | 10 (40%) | 3 (12%) | 0.06 |
Four patients (16%) had AF before septal myectomy. Two of these patients underwent concomitant Maze surgery, 1 of whom did not have recurrence of AF at follow-up. A third patient had undergone a Maze surgery 3 years before septal myectomy, after which he had recurrent AF both before and after septal myectomy. The fourth patient with AF before surgery did not undergo Maze surgery and did not have recurrence of AF in follow-up.
As expected, septal myectomy resulted in a significant reduction in interventricular septal wall thickness (from 2.1 ± 0.5 cm to 1.5 ± 0.3 cm, p <0.001), and posterior wall thickness remained unchanged. LVOT gradient at rest significantly decreased from 52.2 ± 51.3 mm Hg to 18.2 ± 25.0 mm Hg (p <0.001). LA volume index decreased from 47.2 ± 17.6 ml/m 2 to 35.9 ± 17.0 ml/m 2 (p = 0.001). Analysis of diastolic dysfunction was performed in 20 of the 25 patients, excluding those who underwent MVR or mitral annuloplasty repair. Markers of diastolic function significantly improved as well including lateral e′ velocity and lateral E/e′ ratio (from 7.3 ± 2.9 cm/s to 9.8 ± 3.1 cm/s, p = 0.012 and from 14.8 ± 6.3 to 11.7 ± 5.5, p = 0.051, respectively). The septal e′ velocity and septal E/e′ ratio were not included in the overall analysis of diastolic function because myectomy makes this assessment invalid ( Table 2 ).
| Pre-Operative (n = 25) | Follow-Up (n = 25) | p-Value | |
|---|---|---|---|
| LA volume index (ml/m 2 ) | 47.2 ± 17.6 | 35.9 ± 17.0 | 0.001 |
| LV end systolic dimension (cm) | 2.8 ± 0.7 | 3.2 ± 0.7 | 0.007 |
| LV end diastolic dimension (cm) | 4.2 ± 0.5 | 4.5 ± 0.6 | 0.012 |
| Interventricular septal thickness (cm) | 2.1 ± 0.5 | 1.5 ± 0.3 | <0.001 |
| Posterior wall thickness (cm) | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.397 |
| LV mass index | 146.1 ± 50.8 | 118.5 ± 33.2 | <0.001 |
| LV ejection fraction (%) | 64.2 ± 7.5 | 63.2 ± 11.2 | 0.598 |
| E velocity (cm/sec) ∗ | 96.8 ± 27.0 | 98.9 ± 30.4 | 0.782 |
| A velocity (cm/sec) ∗ | 70.9 ± 21.4 | 84.2 ± 27.8 | 0.046 |
| E/A ratio ∗ | 1.4 ± 0.5 | 1.2 ± 0.4 | 0.260 |
| Deceleration time (msec) ∗ | 256.0 ± 64.7 | 226.4 ± 71.0 | 0.257 |
| Lateral e′ velocity (cm/sec) ∗ | 7.3 ± 2.9 | 9.8 ± 3.1 | 0.012 |
| Lateral E/e′ ratio ∗ | 14.8 ± 6.3 | 11.7 ± 5.5 | 0.051 |
| Septal e′ velocity (cm/sec) ∗ | 5.4 ± 1.1 | 20.7 ± 10.9 | <0.001 |
| Septal E/e′ ratio ∗ | 18.4 ± 7.2 | 5.6 ± 1.7 | <0.001 |
| LV outflow tract gradient (mm Hg) | 52.2 ± 51.3 | 18.2 ± 25.0 | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


