There are limited data on the effect of iloprost therapy in patients with Eisenmenger syndrome (ES). The aim of our study was to evaluate the effect of inhaled iloprost therapy on exercise capacity, quality of life (QoL), cardiac function, and hemodynamics in patients with ES. Eighteen consecutive patients with ES and exertional dyspnea according to the World Health Organization functional class III or IV were prospectively recruited. Exercise capacity was assessed by a 6-minute walk test, and QoL was measured on a 12-Item Short-Form Health Survey. Echocardiographic measurements included peak systolic and mean pulmonary arterial pressures, pulmonary vascular resistance, and myocardial performance index of the right ventricle (RV). All patients underwent comprehensive evaluation at baseline and after 24 weeks of treatment. Of the 18 patients with ES, 13 were included for analysis. After 24 weeks of iloprost therapy, 6-minute walk test distance significantly increased (289.1 ± 76.9 to 369.5 ± 93.4 m, p = 0.032) in addition to concomitant improvements in the 12-Item Short-Form Health Survey physical and mental component summaries (20.6 ± 19.3 to 52.6 ± 28.0, p <0.05; 33.9 ± 19.7 to 54.9 ± 21.3, p <0.05, respectively). RV myocardial performance index improved significantly after treatment (0.80 ± 0.31 to 0.59 ± 0.12, p = 0.042). Pulmonary arterial pressure and pulmonary vascular resistance did not improve with iloprost therapy. This study showed that 24 weeks of inhaled iloprost therapy in patients with ES led to significant improvements in exercise capacity, QoL, and RV function. These results likely explain the symptomatic relief reported by patients with ES receiving iloprost therapy.
In recent decades, there have been new developments in the management of pulmonary arterial hypertension (PAH), leading to new classes of drugs including prostacyclin analogs, phosphodiesterase inhibitors, endothelin receptor antagonists, and nitric oxide. In patients with Eisenmenger syndrome (ES), randomized controlled trials have reported favorable short-term and long-term outcomes with bosentan, an on orally active dual endothelin receptor antagonist. In patients with ES and hepatic dysfunction secondary to right-sided cardiac failure, inhaled iloprost or prostacyclin analogs have been proposed as alternative therapeutic options. However, data on the treatment of patients with ES using iloprost remain limited. Therefore, we aimed to determine the effect of inhaled iloprost treatment on exercise capacity, quality of life (QoL), cardiac function, and hemodynamics in patients with ES.
Methods
The Effects of Iloprost Treatment in Adult Patients with Pulmonary Arterial Hypertension Related to Congenital Heart Disease (EIGER) study is a prospective, multicenter, single-arm trial. Patients with exertional dyspnea based on the World Health Organization functional class (WHO FC) III or IV along with Eisenmenger physiology (nonrestrictive intracardiac or extracardiac communication with a right-to-left shunt at rest) were recruited from December 2010 to June 2012.
Patients with age >20 years in addition to congenital heart defects, such as univentricular heart, patent ductus arteriosus, ventricular septal defect, atrial septal defect, or atrioventricular septal defect, or those with persistent PAH after previous closure of a congenital heart disease (CHD) were included for further study. Exclusion criteria included severe left ventricular dysfunction (ejection fraction of ≤40%); pulmonary venous congestion as measured invasively or by echocardiography; obstruction of the right ventricular (RV) outflow tract (RVOT), pulmonary valve, or pulmonary arteries; patients on glibenclamide or cyclosporine treatment; known coronary artery disease; planned surgical procedure during the study period; systolic blood pressure ≤85 mm Hg; inability to perform a 6-minute walk test (6MWT) and/or comply with the study protocol; serum creatinine level of ≤125 μmol/L; patients who had started or discontinued treatment for PAH within 1 month of screening; and anticoagulant use. Stable PAH after completion of the EIGER study was required. Use of phosphodiesterase inhibitors, prostanoids, and investigational endothelin receptor antagonists was not allowed during the study. Women were advised to use reliable contraceptive methods (barrier-type devices, intrauterine devices, or oral contraceptives in combination with barrier methods). The study was conducted according to the most recent amendments to the Declaration of Helsinki and adhered to the Good Clinical Practice Guidelines. The study protocol was approved by the local ethics review committees, and written informed consent was obtained from all patients ( ClinicalTrials.gov identifier: NCT01383083 ).
After baseline examinations, patients with PAH secondary to CHD were treated with inhaled iloprost based on a standardized treatment protocol (described in further detail later). Both at baseline and after 24 weeks of therapy (obligatory period), clinical and functional statuses were evaluated using laboratory tests (including levels of hemoglobin, creatinine, uric acid, and N-terminal pro-B natriuretic peptide, WHO FC, 6-minute walk distance [6-MWD], QoL questionnaires, and Doppler echocardiography).
To examine QoL, a written Korean version of the 12-Item Short-Form Health Survey (SF-12) questionnaire was administered to all patients by a dedicated study nurse. The SF-12 questionnaire is an abbreviated version of the 36-Item Short-Form Health Survey, which has been shown to be useful in various medical specialties without bias for symptoms of a specific disease. The SF-12 questionnaire has high consistency with the 36-Item Short Form Health Survey and has easily measured variables. The SF-12 questionnaire includes 8 categories and 12 questions for the physical component summary including general health, physical functioning, physical role, and bodily pain and for the mental component summary including vitality, emotional role, social functioning, and mental health.
The 6MWT, commonly used as a measure for evaluating treatment effects in PAH, was conducted in a 20-m indoor marked corridor, according to the guidelines of the American Thoracic Society, along with continuous pulse oximetry monitoring. Previous evidence has demonstrated that this test is easy to administer, well tolerated, and accurately reflects activities of daily living. Heart rate, pulse oximetry, dyspnea, and the Borg Scale score were recorded at baseline and after 6 minutes of walking.
All echocardiographic examinations were performed on a GE Vivid 7 ultrasound machine (GE Medical System, Horden, Norway) with a 2.5-MHz transducer. Two-dimensional and M-mode measurements, flow velocities using pulse- and continuous-wave Doppler techniques were performed according to the recommendations of the American Society of Echocardiography. The RVOT time velocity integral (TVI RVOT ; reported in cm) was obtained by positioning the sample volume of the pulse-wave Doppler at the RVOT. Doppler-derived pulmonary artery systolic pressure (reported in mm Hg) was calculated from the maximal tricuspid regurgitation velocity (TR V max ) using the simplified Bernoulli formula. Pulmonary vascular resistance (PVR) was then calculated by the simple index of TR V max /TVI RVOT , which provides a reliable estimation of PVR over a wide range in patients with PAH with various underlying causes. RV function was measured using tricuspid annular plane systolic excursion, RV myocardial performance index (MPI), and RV ejection fraction. All echocardiograms were analyzed offline with an EchoPAC Dimension System (General Electric, Horten, Norway) by an experienced operator (KIC) blinded to the clinical status of the study subjects.
An acceptable target dose of iloprost in adult patients was 2.5 μg administered 4 to 6 times/day, and the therapeutic dose was titrated according to patient compliance. Given safety and tolerability concerns, patients received 2.5 μg twice daily during the first 4 weeks of treatment. After 4 weeks of therapy, therapeutic dose was increased to the target level (10 μg) if iloprost was well tolerated. As part of our safety monitoring, arterial blood pressure, heart rate, and oxygen saturation (estimated with pulse oximetry after 5 minutes of rest) were measured during every visit. Patients who did not tolerate target dosing received a lower daily dose (once or twice daily) with a target dose titrated up from the original dose.
SF-12 scores were assessed at baseline and reassessed after 24 weeks of therapy. Clinical examination, 6MWT, laboratory testing, and transthoracic echocardiography were also performed after treatment.
Statistical analysis was performed with SPSS for Windows, version 12.0 (SPSS Inc., Chicago, Illinois). Results are presented as mean ± SD or as percentage. Study end points included changes in SF-12 scores, 6-MWD, PVR, and WHO FC, as measured at baseline and after 24 weeks of treatment with inhaled iloprost. Comparisons were performed using Student t test for paired quantitative variables and chi-square or Fisher’s exact test for qualitative variables. Nonparametric Mann-Whitney U test was performed to identify differences between baseline measurements and after 24 weeks of treatment. Correlations between variables were assessed by calculating the Pearson correlation coefficient. Statistical significance threshold was set at p <0.05.
Results
Eighteen patients with Eisenmenger physiology were recruited during the study period ( Table 1 ). Mean age was 45 ± 11 years (range, 30 to 69 years), and men constituted 67% of the study population. Most patients (89%) had isolated cardiac lesions—with the most common being ventricular septal defect (n = 8), patent ductus arteriosus (n = 4), and atrial septal defect (n = 4)—whereas 2 patients had complex CHD. All patients experienced dyspnea, had impaired exercise tolerance, and were in WHO FC III (67%) or IV (33%) at baseline examination. During the study, 3 patients were prematurely discontinued from study at days 5, 60, and 90 because of adverse events such as dizziness (n = 1) and hoarseness (n = 2). Additionally, 2 patients were discontinued from the study because the health insurance company refused to reimburse medication. Overall, these 5 patients were not considered in the subsequent analysis. In total, 13 patients tolerated inhaled iloprost therapy throughout the entire study period without major adverse effects or significant decrease in arterial oxygen saturation. Eleven patients achieved target dose, and 2 patients did not tolerate the increase, and the end doses were 2.5 μg twice daily. No additional symptoms or laboratory abnormalities were seen after 24 weeks of treatment. Also, there were no clinically significant changes in vital signs (pulse rate, systolic and diastolic blood pressures, or body weight). No patients required additional therapy because of disease progression during the study period.
| Characteristics | ES (n = 18) |
|---|---|
| Age (yrs) | 45 ± 11 |
| Men (%) | 12 (67) |
| Duration (mo) | 132 ± 888 |
| WHO FC (%) | |
| III | 12 (67) |
| IV | 6 (33) |
| Previous PAH-specific drug (%) | 8 (44) |
| Digoxin (%) | 5 (28) |
| Diuretics (%) | 11 (61) |
| Warfarin (%) | 4 (22) |
| Systolic blood pressure (mm Hg) | 113 ± 15 |
| Diastolic blood pressure (mm Hg) | 69 ± 11 |
| Heart rate (beats/min) | 81 ± 13 |
| Hemoglobin (mg/dl) | 17 ± 3 |
| Uric acid (mg/dl) | 8 ± 3 |
| Mean N-terminal pro-B natriuretic peptide (ng/L) | 1,135 ± 1,176 |
| Serum creatine level (mg/dl) | 0.9 ± 0.3 |
| 6-MWD (m) | 290 ± 96 |
| Oxygen saturation (%) | 90 ± 7 |
| SF-12 questionnaire, physical role score | 21 ± 18 |
| SF-12 questionnaire, emotional role score | 35 ± 17 |
| Stroke volume (ml) | 53 ± 16 |
| Cardiac output (L/min) | 4.0 ± 1.2 |
| Mean pulmonary artery pressure (mm Hg) | 55 ± 18 |
| Pulmonary artery systolic pressure (mm Hg) | 98 ± 18 |
| PVR (dynes·s·cm −5 ) | 4.5 ± 1.3 |
| Left ventricular ejection fraction (%) | 65 ± 5.8 |
| Ratio of mitral valve to mitral annular early diastolic velocity | 9.6 ± 3.8 |
| Left atrial volume index (mL/m 2 ) | 24 ± 4.8 |
| RV fractional area change (%) | 36 ± 8 |
| RV MPI | 0.8 ± 0.3 |
| Tricuspid annular plane systolic excursion | 17 ± 2.5 |
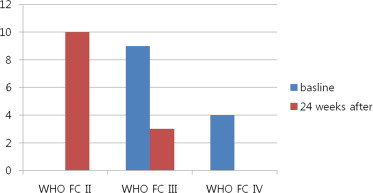
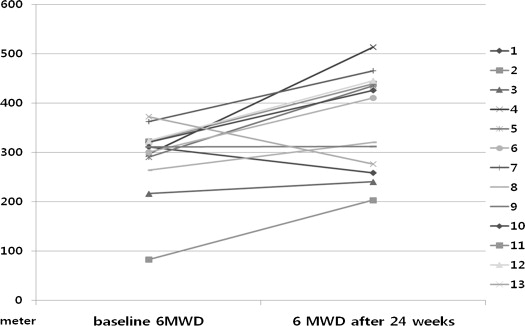
At baseline, all patients were in WHO FC III or IV. After 24 weeks of treatment, 10 patients improved from WHO FC III (n = 6) or WHO FC IV (n = 4) to WHO FC II and 3 patients remained stable at WHO FC III ( Figure 1 ). No patients experienced a decrease in WHO FC. Table 2 lists the changes in clinical parameters between baseline evaluation and after 24 weeks of inhaled iloprost therapy. Six-minute walk test distance improved significantly (p = 0.032), with a distance increase of 75.0 ± 85.7 m from baseline ( Figure 2 ). All components of the SF-12 improved significantly after 24 weeks of treatment, with concomitant improvements in the SF-12 physical and mental component summaries ( Table 3 and Figure 3 ). There were no significant changes in echocardiographic parameters other than a significant improvement in RV MPI (p = 0.042; Table 4 ). Improvement of SF-12 components did not correlate with the change of PVR, mean pulmonary arterial pressure, or RV MPI ( Table 5 ). Improvement of SF-12 components (physical and mental component summaries) significantly correlated with improvements in 6-MWD. N-terminal pro-B natriuretic peptide levels remained unchanged after treatment.
| Parameters | Baseline (n = 13) | After 24 Weeks (n = 13) | p |
|---|---|---|---|
| Systolic blood pressure (mm Hg) | 111 ± 15 | 105 ± 16 | 0.366 |
| Diastolic blood pressure (mm Hg) | 70 ± 12 | 62 ± 13 | 0.142 |
| Heart rate (beats/min) | 82 ± 10 | 81 ± 11 | 0.751 |
| Hemoglobin level (mg/dl) | 17 ± 3.0 | 17 ± 2.9 | 0.676 |
| Uric acid level (mg/dl) | 8.8 ± 3.0 | 7.1 ± 2.1 | 0.131 |
| Mean N-terminal pro-B natriuretic peptide (ng/L) | 1,176 ± 1,210 | 960 ± 1,124 | 0.654 |
| Serum creatine level (mg/dl) | 0.98 ± 0.28 | 0.90 ± 0.25 | 0.499 |
| 6-MWD (m) | 289 ± 77 | 370 ± 93 | 0.032 |
| Oxygen saturation (%) | 90 ± 7.6 | 90.6 ± 8.37 | 0.840 |
| Baseline (n = 13) | After 24 Weeks (n = 13) | p | |
|---|---|---|---|
| SF-12 physical component summary | 21 ± 19 | 53 ± 28 | 0.004 |
| Physical function | 15 ± 20 | 40 ± 33 | 0.036 |
| Physical role | 22 ± 23 | 54 ± 37 | 0.020 |
| Bodily pain | 35 ± 31 | 73 ± 33 | 0.009 |
| General health | 10 ± 17 | 44 ± 19 | <0.001 |
| SF-12 mental component summary | 34 ± 20 | 55 ± 21 | 0.019 |
| Vitality | 19 ± 19 | 44 ± 32 | 0.032 |
| Social functioning | 40 ± 33 | 67 ± 42 | 0.092 |
| Emotional role | 41 ± 28 | 69 ± 30 | 0.028 |
| Mental health | 37 ± 16 | 41 ± 17 | 0.536 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


