Enhanced external counterpulsation (EECP) is a noninvasive technique for treatment of symptomatic coronary artery disease in patients not amenable to revascularization procedures. However, the mechanisms underlying the benefits of EECP remain unknown. We hypothesized that decreases in arterial stiffness and aortic wave reflection are a therapeutic target for EECP. Patients with coronary artery disease and chronic angina pectoris were randomized (2:1 ratio) to 35 1-hour sessions of EECP (n = 28) or sham EECP (n = 14). Central and peripheral arterial pulse-wave velocity and aortic wave reflection (augmentation index) were measured using applanation tonometry before, and after 17 and 35 1-hour treatment sessions. Wasted left ventricular pressure energy and aortic systolic tension–time index, markers of left-ventricular myocardial oxygen demand, were derived from the synthesized aortic pressure wave. Exercise duration, anginal threshold, and peak oxygen consumption were measured using a graded treadmill test. Central arterial stiffness and augmentation index were decreased after 17 and 35 sessions in the treatment group. Measurements of peripheral arterial stiffness were decreased after 35 sessions in the treatment group. Changes in aortic pressure wave reflection resulted in decreased measurements of myocardial oxygen demand and wasted left ventricular energy. No changes in central or peripheral arterial stiffness were observed in the sham group. Furthermore, measurements of exercise capacity were improved in the EECP group but unchanged in the sham group. In conclusion, EECP therapy decreases central and peripheral arterial stiffness, which may explain improvements in myocardial oxygen demand in patients with chronic angina pectoris after treatment.
Angina pectoris can be caused by decreased coronary blood supply or increased myocardial oxygen demand. Enhanced external counterpulsation (EECP) has been demonstrated to be an effective treatment for patients with angina pectoris. The central hypothesis in most investigations conducted to elucidate the mechanism of action is that EECP may promote coronary angiogenesis and improved myocardial perfusion. Despite evidence of improved myocardial perfusion in dogs, there is a lack of data that support the central hypothesis in humans. A few small nonrandomized studies have reported that improvements in anginal class and exercise capacity are a result of improved myocardial perfusion. However these findings have not been entirely consistent, thus suggesting extracardiac factors such as altered peripheral vascular function and myocardial oxygen demand may be the therapeutic target for EECP. Therefore, in the present randomized sham-controlled study we tested the hypothesis that EECP treatment would decrease central and peripheral arterial stiffness and improve indexes of myocardial oxygen consumption in patients with coronary artery disease (CAD) and chronic angina pectoris.
Methods
Forty-two consecutive patients with chronic stable angina referred for EECP treatment were randomized in a 2:1 manner into an EECP treatment group or a sham-EECP control group. Patients were enrolled for EECP treatment from November 2004 through June 2009 because they had chronic angina for >3 months caused by myocardial ischemia in the presence of angiographic multivessel CAD that could not be controlled by a combination of medical therapy, angioplasty/stent, and/or coronary bypass surgery. Patient recruitment was relatively slow because of the single-center study design and the nonmetropolitan regional location of Shands Hospital at the University of Florida. The study was approved by the institutional review board of the University of Florida and written informed consent was obtained from all patients. Patient characteristics are presented in Table 1 .
| Variable | EECP | Sham |
|---|---|---|
| (n = 28) | (n = 14) | |
| Age (years) | 64 ± 2 | 64 ± 3 |
| Men | 22 (79%) | 12 (86%) |
| Height (cm) | 173 ± 2 | 174 ± 2 |
| Weight (kg) | 92.6 ± 3 | 101 ± 3 |
| Body mass index (kg/m 2 ) | 30.9 ± 0.80 | 33.4 ± 1.1 |
| Left ventricular ejection fraction (%) | 51.6 ± 2.8 | 48.2 ± 3.9 |
| Left ventricular ejection fraction <40% | 5 (18%) | 2 (14%) |
| Previous coronary artery bypass graft | 19 (68%) | 11 (79%) |
| Previous percutaneous transluminal coronary angioplasty | 23 (82%) | 12 (86%) |
| Previous myocardial infarction | 17 (61%) | 6 (43%) |
| Multivessel coronary artery disease | 25 (89%) | 13 (93%) |
| Diabetes mellitus | 21 (75%) | 11 (79%) |
| Hypertension | 23 (82%) | 12 (86%) |
| Hyperlipidemia | 26 (93%) | 14 (100%) |
| Lipid-lowering agent | 28 (100%) | 14 (100%) |
| β Blocker | 24 (86%) | 11 (79%) |
| Calcium channel blocker | 11 (39%) | 6 (43%) |
| Long-lasting nitrates | 25 (89%) | 12 (86%) |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 26 (93%) | 12 (86%) |
| Insulin | 4 (14%) | 2 (14%) |
Exclusion criteria included absence of ST-segment depression (1 mm minimum) during exercise testing, coronary artery bypass grafting within previous 3 months or percutaneous coronary intervention in previous 6 months, arrhythmia that would significantly interfere with triggering of the EECP device, symptomatic heart failure and/or left ventricular ejection fraction <30%, valvular heart disease, implantable cardioverter–defibrillator if triggered within previous 6 months, history of deep vein thrombosis, uncontrolled hypertension, pregnancy, pulmonary congestion, and systemic hypotension.
Patients in the EECP (n = 28) and sham (n = 14) groups received 35 1-hour daily sessions of EECP for 7 consecutive weeks using cuff inflation pressures of 300 and 70 mm Hg, respectively. It was previously determined that 70 mm Hg inflation pressure is adequate to preserve the appearance and feel of EECP application but insufficient to alter blood pressure (BP). The EECP equipment (Vasomedical, Westbury, New York) has been described previously.
Data were collected and analyzed at study entry and after 17 and 35 1-hour sessions of EECP or sham EECP. All measurements were performed in a quiet temperature-controlled room (21°C to 23°C) with subjects in a fasting state (10 to 12 hours). Subjects were asked to abstain from caffeine and alcohol for ≥24 hours before vascular measurements. To avoid potential diurnal variations, all measurements were conducted in the morning (7:00 to 9:00 a.m. ). All patients discontinued their medications for 12 hours before vascular measurements.
After 15 minutes of supine rest, heart rate and brachial BP measurements were performed in triplicate in the left arm using an automated noninvasive BP cuff (Omron, Inc., Bannockburn, Illinois). An average of the 3 heart rate and BP measurements were used for values at rest. Assessment of arterial wave reflection characteristics was performed noninvasively using the SphygmoCor system (AtCor Medical, Sydney, Australia) described previously. Briefly, peripheral pressure waveforms were recorded from the radial artery at the wrist using applanation tonometry with a high-fidelity micromanometer (Millar Instruments, Houston, Texas). A validated generalized transfer function was used to generate the corresponding aortic pressure waveform.
Pulse-wave analysis of aortic pressure waveform provided the following key variables of interest: aortic augmentation index, round trip travel time of forward traveling wave from ascending aorta to major reflection site and back, and wasted left ventricular pressure energy, which is a component of extra myocardial oxygen requirement because of early systolic wave reflection. Wasted left ventricular pressure energy was estimated as ([π/4] × [augmented pressure × systolic duration of reflected wave] × 1.333), where 1.333 is the conversion factor for millimeters of mercury per second to dynes per square centimeter per second. Augmented pressure is defined as the difference between the first (forward wave) and second systolic shoulders of aortic systolic BP. Additional calculations derived from the synthesized aortic pressure wave were the aortic systolic tension–time index (AsTTI), diastolic pressure–time index, and subendocardial viability ratio. AsTTI is a marker of aortic systolic stress and myocardial oxygen demand and was estimated as the integral of aortic pressure and time during ventricular systole. Diastolic pressure–time index is an indirect indicator of diastolic perfusion and was estimated as the integral of diastolic pressure during ventricular diastole. Subendocardial viability ratio is the ratio of diastolic pressure–time index to TTI and an index of subendocardial perfusion. Only high-quality recordings, defined as an in-device quality index of >80%, were accepted for analysis.
To determine pulse-wave velocity (PWV), pressure waveforms were recorded at the following 3 sets of sites sequentially—carotid–radial, carotid–femoral, and femoral–dorsalis pedis—using a SphygmoCor Pulse Wave Velocity Vx system and SCOR-2000 6.31 software (AtCor Medical). Pressure waveforms were gated with simultaneous electrocardiography and used to calculate PWV between the respective sites. Foot-to-foot PWV to each peripheral site (radial, femoral, dorsalis pedis) was calculated by determining the delay between the appearance of each pressure waveform foot in the carotid and peripheral sites. The distance between recording sites was adjusted for parallel transmission in the aorta and carotid by correcting for the distance between the suprasternal notch and the carotid. These corrected distances were divided by the respective foot-to-foot transmission delays (carotid–radial, carotid–femoral) to produce PWV. Central PWV (in the mostly elastic aorta) was evaluated using the carotid–femoral data and peripheral PWV (in the more muscular conduits) using the femoral–dorsalis pedis and carotid–radial data.
All subjects performed a symptom-limited maximum graded exercise test on a treadmill using a modified Naughton protocol with a metabolic cart for peak oxygen uptake measurements at study entry and after 17 and 35 hours of treatment. Primary measurements included time to angina, total exercise duration, and peak oxygen uptake. The Seattle Angina Questionnaire was used to measure anginal symptomatology. In addition, Canadian Cardiovascular Society anginal classification was determined.
All statistical analyses were performed using SPSS 18.0 for Windows (SPSS, Inc., Chicago, Illinois). Group and continuous variable data are presented as mean ± SEM. All data were tested for normal distribution with the Shapiro–Wilk test for normality. An alpha level of p <0.05 was required for statistical significance. Repeated measurements analysis of variance was used to evaluate all continuous dependent variables. When a significant group-by-time interaction was observed, within-group comparisons between time points and between-group comparisons at each time point were performed using Tukey post hoc analysis.
Results
All patients completed the entire EECP or sham treatment sessions. Baseline characteristics for the EECP and sham subjects are presented in Table 1 . EECP and sham groups did not differ with respect to age, gender, height, weight, body mass index, drug therapy, cardiovascular history and/or previous procedures, or cardiovascular risk factors. None of the patients were current smokers. Patients did not change their daily medication or physical activity during the duration of the study.
Hemodynamic and pulse-wave analysis results are presented in Table 2 . Baseline (before) hemodynamic and pulse-wave characteristics did not differ between the EECP and sham groups ( Table 2 ). Mean arterial pressure and aortic systolic pressure were decreased after 17 sessions of EECP treatment. Systolic, diastolic, and pulse pressures in the periphery (brachial artery) and aorta were decreased after 35 sessions of EECP. There were no significant changes in any of the BP components in the sham group.
| Variable | EECP (n = 28) | Sham (n = 14) | ||||
|---|---|---|---|---|---|---|
| PRE | MID | POST | PRE | MID | POST | |
| Heart rate (beats/min) | 59 ± 2 | 60 ± 2 | 61 ± 2 | 60 ± 2 | 62 ± 2 | 61 ± 2 |
| Mean arterial pressure (mm Hg) | 95 ± 2 | 92 ± 2 ⁎ § | 90 ± 2 † § | 97 ± 3 | 99 ± 3 | 97 ± 2 |
| Brachial systolic pressure (mm Hg) | 135 ± 4 | 131 ± 4 § | 127 ± 3 † § | 137 ± 3 | 139 ± 4 | 135 ± 3 |
| Brachial diastolic pressure (mm Hg) | 76 ± 2 | 74 ± 1 | 73 ± 2 ⁎ § | 78 ± 3 | 79 ± 3 | 78 ± 2 |
| Brachial pulse pressure (mm Hg) | 59 ± 4 | 57 ± 3 | 54 ± 3 † § | 59 ± 4 | 60 ± 3 | 59 ± 4 |
| Aortic systolic pressure (mm Hg) | 125 ± 4 | 120 ± 4 ⁎ § | 116 ± 3 † § | 127 ± 3 | 128 ± 4 | 126 ± 3 |
| Aortic diastolic pressure (mm Hg) | 76 ± 2 | 74 ± 1 § | 73 ± 2 ⁎ § | 78 ± 3 | 80 ± 3 | 78 ± 2 |
| Aortic pulse pressure (mm Hg) | 48 ± 3 | 45 ± 3 | 42 ± 3 † § | 48 ± 4 | 47 ± 3 | 47 ± 3 |
| Augmented pressure (mm Hg) | 15 ± 2 | 13 ± 2 | 11 ± 2 † ‡ § | 15 ± 2 | 14 ± 2 | 15 ± 2 |
| Round trip travel time of pressure wave from heart to periphery and back (ms) | 140 ± 2 | 143 ± 2 † § | 145 ± 2 † § | 139 ± 3 | 139 ± 3 | 138 ± 3 |
| Systolic duration of reflected wave (ms) | 204 ± 6 | 196 ± 6 | 188 ± 6 † ‡ | 197 ± 7 | 192 ± 5 | 193 ± 7 |
† p <0.01 versus pretreatment values.
‡ p <0.05 versus midway values.
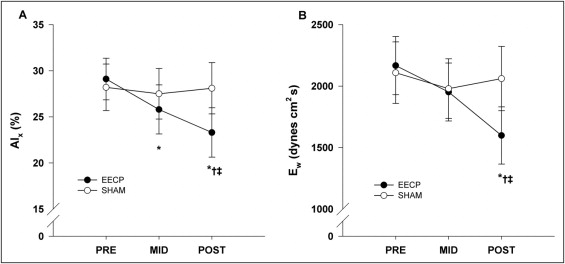
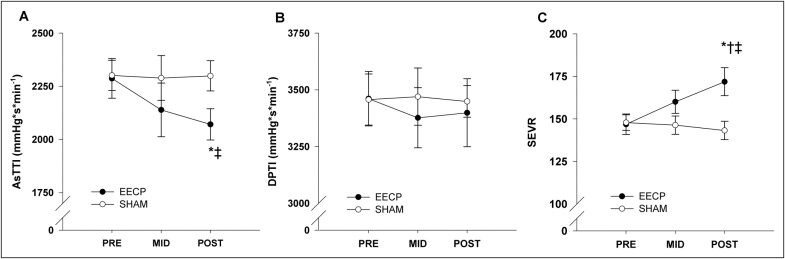
Pulse-wave analysis results demonstrated a progressive decrease in aortic augmentation index after 17 (25.8 ± 2.7%) and 35 (23.3 ± 2.7%) sessions of EECP compared to baseline (29.1 ± 2.3%, p ≤0.01; Figure 1 ). Likewise, there was a progressive increase in round trip travel time of pressure wave from the heart to the periphery and back after 17 and 35 sessions of EECP compared to baseline ( Table 2 ). In addition, there were decreases in augmented pressure (reflected pressure wave amplitude) and systolic duration of reflected wave after 35 sessions of EECP ( Table 2 ). In turn this lead to a substantial decrease in wasted left ventricular pressure energy after 35 sessions of EECP compared to pretreatment values (1,598 ± 233 vs 2,167 ± 372 dyne/cm 2 /s, p <0.01; Figure 1 ). AsTTI was also decreased after 35 sessions of EECP (2,071 ± 73 vs 2,287 ± 93, p <0.05; Figure 2 ). There were no differences in diastolic pressure–time index (3,399 ± 150 vs 3,461 ± 120, p = 0.68; Figure 2 ), but the difference in AsTTI resulted in a significantly higher subendocardial viability ratio (172 ± 8 vs 147 ± 6, p <0.01; Figure 2 ) after treatment in the EECP group. No changes in BP (peripheral or aortic) and indexes of wave reflection were observed in the sham group.
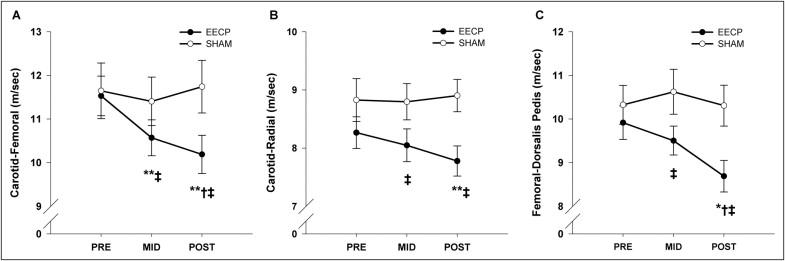
Central and peripheral PWV values at baseline and after 17 and 35 sessions of EECP and sham EECP are presented in Figure 3 . Regional PWV did not differ between groups before the respective treatments. Carotid–femoral (central) PWV was decreased after 17 (10.6 ± 0.4 m/s, p <0.01) and 35 (10.2 ± 0.4 m/s, p <0.01) sessions of EECP compared to pretreatment values (11.5 ± 0.5 m/s; Figure 3 ). Carotid–radial (7.8 ± 0.3 vs 8.3 ± 0.3 m/s, p <0.01; Figure 3 ) and femoral–dorsalis pedis (8.7 ± 0.4 vs 9.9 ± 0.4 m/s, p <0.05; Figure 3 ) PWVs (peripheral) were decreased after 35 sessions of EECP compared to pretreatment values. No changes in central or peripheral PWV were observed in the sham group.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


