Omega-3 polyunsaturated fatty acids in fish oils may have antifibrillatory effects; however, their electrophysiologic effects in paroxysmal atrial fibrillation (PAF) remain unknown. The aim of this study was to investigate the effects of chronic fish oil supplementation on human pulmonary vein (PV) and left atrial electrophysiology in PAF. Patients with PAF undergoing PV isolation were randomized ≥1 month before their procedure into a control group (n = 18) or a fish oil group (n = 18) in an unblinded fashion. The latter were supplemented with fish oil 6 g/day for a mean of 40 ± 12 days. Pulmonary venous and left atrial effective refractory periods (ERPs), PV conduction, and susceptibility to AF initiated within PVs were assessed. Compared to the control group, the fish oil group had (1) longer left-sided (p = 0.002) and right-sided (p = 0.001) pulmonary venous ERPs; (2) less dispersion of pulmonary venous ERPs (left PVs p = 0.001, right PVs p = 0.07); (3) longer left atrial ERPs (p = 0.02); (4) no difference in pulmonary venous conduction; (5) lower incidence of AF initiated from PVs during ERP testing (77% vs 31%, p = 0.02); and (6) prolongation of mean AF cycle length (p = 0.009) and shortest AF cycle length in PVs (p = 0.04). In conclusion, patients with PAF chronically supplemented with fish oils exhibit distinctive electrophysiologic properties including prolonged pulmonary venous and left atrial ERPs and decreased susceptibility to initiation AF from within PVs. These changes may in part explain the antifibrillatory effect of chronic omega-3 polyunsaturated fatty acid supplementation in patients with PAF.
Omega-3 polyunsaturated fatty acids in fish oils have consistently been shown to have antifibrillatory effects in preclinical studies. In humans, epidemiologic, observational, and randomized studies on incident, paroxysmal, and postoperative atrial fibrillation (AF) with acute or chronic fish oil exposure have been contradictory. Pulmonary vein (PV) ectopy is critical for the initiation and maintenance of paroxysmal AF (PAF). In addition, PVs of patients with PAF exhibit distinct electrophysiologic properties that may form the “substrate” for re-entry. The effect of chronic fish oil supplementation on human PV electrophysiology remains unknown. Chronic fish oil supplementation prolongs right atrial and coronary sinus refractoriness and decreases vulnerability to inducible AF; however, this was assessed in patients without structural heart disease or clinical AF. The aim of this study was to evaluate the effect of chronic polyunsaturated fatty acid supplementation on pulmonary venous and left atrial electrophysiology in patients with PAF.
Methods
Patients 18 to 75 years old with PAF scheduled to undergo PV isolation were recruited. Patients on amiodarone, fish intake >1 time/week, or previous fish oil supplementation were excluded. A 7-day Holter monitor and Atrial Tachyarrhythmia Symptom Severity Scale were performed before recruitment. At least 1 month before ablation, patients were randomized to active therapy with fish oil (fish oil group) or no therapy (control group). Fish oil 6 g/day containing a total dose of docosahexaenoic acid (DHA) 1.5 g and eicosapentaenoic acid (EPA) 0.3 g were prescribed (Nu-mega Ingredients Pty. Ltd., Victoria, Australia). A DHA-enriched preparation was chosen as it may produce more potent antifibrillatory effects than EPA. A minimum exposure of 30 days was mandatory because atrial enrichment with EPA and DHA reaches a maximum at this time. Patients had continuous electrocardiographic monitoring for 4 hours before ablation; patients with AF >1 minute were excluded from the study to eliminate the possible confounding effect of AF on atrial and pulmonary venous refractoriness.
The operator and technical staff were blinded to patient groups. Off-line analysis was performed at a later date by 2 authors who were also blinded to patient group assignment. Compliance with fish oil therapy was monitored weekly using pill count and by plasma fatty acid profile on the day of ablation. Written informed consent was obtained from each patient, and the Melbourne health research ethics committee approved the protocol.
Blood samples were collected on the day of procedure and centrifuged to separate plasma from the erythrocyte fraction and analyzed as described previously. Total omega-3, omega-6, EPA, and DHA levels were expressed as percent fraction of total fatty acids in the phospholipid fraction.
Our method of PV isolation using general anesthesia, double transseptal punctures, and a single circular mapping catheter has been previously described in detail. Intracardiac catheters were positioned as follows: (1) 10-pole coronary sinus catheter (2- × 5- × 2-mm interelectrode spacing) with the proximal bipole positioned at the coronary sinus ostium as determined in the best septal left anterior oblique projection (6Fr “Response” DEC CSL, St. Jude Medical, Minneapolis, Minnesota); (2) His-bundle electrographic catheter (6Fr “Supreme” Hex JSN, St. Jude Medical), (3) mapping/ablation catheter (7Fr Therapy Cool Path M Curve, St. Jude Medical), and (4) multipolar circular mapping catheter (20-mm Lasso, 7Fr deflectable, Biosense Webster, Diamond Bar, California). Selective pulmonary angiography was used to define the PV antrum; this was followed by creation of left atrial geometry using a 3-dimensional electroanatomical mapping system (NavX, St. Jude Medical). The research protocol consisted of the following items measured in the same sequence in every patient: (1) RR intervals, (2) P-wave duration, (3) pacing threshold, (4) effective refractory periods (ERPs) from the left superior, right superior PVs, posterior left atrium, and distal coronary sinus at 600- and 450-ms drive trains, (5) PV conduction at the same drive trains, and (6) left atrial conduction.
P-wave duration in sinus rhythm was measured on lead II of the surface electrocardiogram and averaged over 10 beats as a surrogate marker of interatrial conduction time. Pacing threshold was determined at each site at a 2-ms pulse width. The catheter was repositioned if a diastolic threshold <5 mA could not be obtained. All measurements were performed at twice the diastolic threshold. ERPs were measured using pacing drive train of 8 beats followed by a single extrastimulus commencing at a coupling interval of 150 ms and incrementing by 10 ms until local capture was demonstrated. For measurement of ERPs and conduction, the ablation catheter was positioned 1 to 2 cm distal to the PV ostium as defined by pulmonary venography and pacing was performed at the floor of the vein as defined by venographic landmarks and assisted with fluoroscopy. Dispersion of refractoriness, an important marker of AF vulnerability, was evaluated using the coefficient of variation of ERP at the 2 cycle lengths (SD/mean × 100%). If AF >1 minute was induced during ERP testing, this was documented and no further assessment of ERP or conduction was attempted.
During AF, the circular mapping catheter was positioned within the PVs for measurement of AF cycle length. Total duration, mean AF cycle length, shortest AF cycle length, and longest AF cycle length in PVs were measured at a sweep speed of 100 mm/s by averaging 30 consecutive cycles. Shortest and longest AF cycle lengths were measured within a random 10-second window of induced AF of >1-minute duration. Only the first induction of AF was used to compare the cycle length between groups.
PV conduction was measured as the conduction time from the pacing site within PVs (1 to 2 cm distal to the os, as described earlier) to the earliest atrial electrogram recorded on the circular mapping catheter positioned at the junction of the PV and left atrium. Left atrial activation time was calculated by pacing the distal coronary sinus and measuring activation time to the atrial electrogram at the His bundle region. Conduction was measured at cycle lengths of 600 and 450 ms after stable capture for ≥10 seconds. Conduction time was determined 5 times at each cycle length and averaged. Autonomic blockade was not administered to not interfere with the treatment of the clinical arrhythmia.
The study intended to detect ≥10-ms difference (SD 10) in pulmonary venous ERPs with 80% power and an alpha level of 5%, producing an estimate of 16 patients required in each group. SPSS 15.0 for Windows (SPSS, Inc., Chicago, Illinois) was used for analysis. To test for associations between categorical variables, chi-square test or Fisher’s exact test was used. Mean values were compared using Student’s t test. Mann–Whitney U or Kruskal-Wallis tests were used for continuous variables where normal distribution was not present. A 2-tailed p value <0.05 was considered statistically significant.
Results
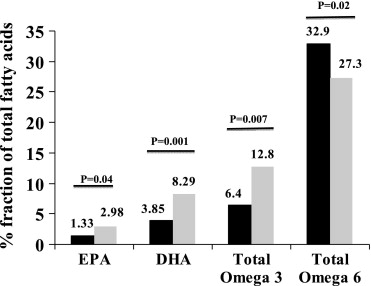
Forty-two patients were screened; 2 were excluded because they were already on fish oil, 2 were taking amiodarone, and another 2 had >1 minute of AF in the 4 hours before the procedure. There were no dropouts in the fish oil group. Thirty-six patients (18 controls, 18 fish oil subjects) constituted the study population (mean age 54 ± 10 years, 72% men). Baseline characteristics were similar ( Table 1 ). Mean duration of fish oil therapy was 40 ± 13 days (range 30 to 66 days). Plasma EPA, DHA, and total omega-3 levels were 2.2-, 2.6-, and 2-fold higher in the fish oil group on the day of testing ( Figure 1 ).
| Variable | Fish Oil (n = 18) | Control (n = 18) | p Value |
|---|---|---|---|
| Age (years) | 56 ± 9 | 51 ± 10 | 0.17 |
| Men | 72% | 72% | 1.0 |
| Duration of atrial fibrillation (years) | 5 ± 4 | 6 ± 5 | 0.71 |
| Number of failed antiarrhythmics | 2 ± 0.9 | 2 ± 0.8 | 0.84 |
| Hypertension | 24% | 28% | 1.0 |
| Left atrial size (cm) | 4 ± 3.8 | 4.1 ± 3 | 0.6 |
| Left atrial area (cm 2 ) | 21 ± 5 | 21 ± 4 | 0.91 |
| Left ventricular ejection fraction (%) | 65 ± 1 | 64 ± 4 | 0.38 |
| Left superior pulmonary vein diameter (mm) | 18 ± 6 | 17 ± 4 | 0.55 |
| Right superior pulmonary vein diameter (mm) | 17 ± 4 | 19 ± 4 | 0.36 |
| Atrial fibrillation symptom total score ⁎ | 9 ± 4 | 8 ± 3 | 0.7 |
| Atrial fibrillation symptom severity score | 17 ± 11 | 14 ± 7 | 0.36 |
| Atrial fibrillation symptom frequency score | 37 ± 10 | 32 ± 8 | 0.2 |
| Number of episodes of atrial fibrillation/month ⁎ | 15 ± 32 | 10 ± 10 | 0.56 |
| Duration of atrial fibrillation episodes (hours) ⁎ | |||
| Minimum | 2.7 ± 2.7 | 2.9 ± 3.5 | 0.87 |
| Maximum | 15 ± 19 | 15 ± 14 | 0.96 |
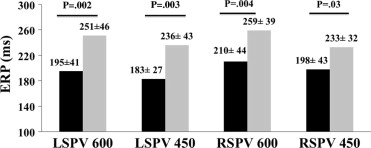
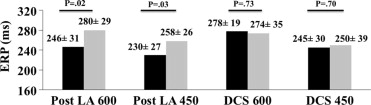
Mean RR interval of the 2 groups was similar before protocol commencement (fish oil 879 ± 290 ms vs control 964 ± 210 ms, p = 0.36). Pulmonary venous threshold was similar in the left superior (fish oil 2.4 ± 0.7 V vs control 2.1 ± 0.4 V, p = 0.33) and right superior (fish oil 2.8 ± 0.4 V vs control 2.9 ± 0.3 V, p = 0.75) PVs, posterior left atrium (fish oil 4.2 ± 0.7 V vs control 4.4 ± 0.7 V, p = 0.63), and distal coronary sinus (fish oil 2.9 ± 0.4 V vs control 2.9 ± 0.3 V, p = 0.93). The fish oil group had significantly longer pulmonary venous and left atrial ERPs at the 2 pacing cycle lengths compared to controls ( Figures 2 and 3 ). There was less dispersion of refractoriness in the PVs of fish oil patients compared to controls (600 ms 5.5 ± 3.7% vs 18.9 ± 11.3%, p = 0.001; 450 ms 9.7 ± 9.8% vs 18.2 ± 10%, p = 0.07). In the control population, ERPs recorded from the left superior and right superior PVs were as short as 120 ms, whereas all patients in the fish oil group had an ERP ≥200 ms. P-wave duration and pulmonary venous and left atrial conduction times were not significantly different between groups ( Table 2 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


