This study was designed to determine the long-term safety and efficacy of using modified double-disk occluders for perimembranous ventricular septal defect (pmVSD) closure in adults. From January 2004 to December 2014, 337 adults with pmVSDs were treated through transcatheter intervention using 2 types of double-disk occluders; 302 patients received a symmetrical concentric pmVSD occluder, and 35 patients received an asymmetrical concentric pmVSD occluder. All patients were followed up through electrocardiography and transthoracic echocardiography until June 2015. The success rate was 100% for both procedures. During the median 71-month follow-up period, no cases of infective endocarditis, cerebrovascular accidents, heart failure, or death occurred. Two major adverse events (0.6%) were recorded: complete atrioventricular block requiring surgical treatment in one patient and severe tricuspid valvular regurgitation requiring surgical repair in another patient. Cardiac conduction block was the most common minor adverse event. The mean left ventricular (LV) end-diastolic volume decreased from 96.6 ± 23.2 ml before intervention to 86.0 ± 22.0 ml (p <0.05) at the 6-month follow-up visit. Previously enlarged LV chambers decreased to normal sizes during the follow-up period. In conclusion, transcatheter closure of pmVSDs using modified double-disk occluders was both safe and effective and yielded excellent long-term results in adults. The potential benefits of this intervention included remodeling of the heart, a reduced incidence of infective endocarditis and prevention of LV volume overload.
Ventricular septal defects (VSDs) are a common congenital cardiac malformation. Up to 80% of these defects are perimembranous VSDs (pmVSDs), which are more common in patients from Asian countries. Because hemodynamically and clinically important pmVSDs are usually treated in childhood, pmVSDs left untreated are believed to be minor lesions that will not require treatment. However, some untreated pmVSDs require VSD closure in adulthood because of a hemodynamically important left-to-right shunt (Qp/Qs >1.5) that causes left heart volume overload, resulting in heart failure, reduced exercise capacity, recurrent bacterial endocarditis, pulmonary hypertension, or atrial fibrillation. Transcatheter closure of a pmVSD using either an Amplatzer pmVSD occluder or a modified double-disk pmVSD occluder is a relatively new technique that has yielded promising results and patient outcomes. However, few studies have focused on the results and outcomes of transcatheter closure of pmVSDs in adults. On the basis of our previous studies in children, as well as on the results of a randomized controlled trial, we designed and completed this retrospective study to evaluate the long-term safety and efficacy of transcatheter closure of congenital pmVSDs in adults.
Methods
From January 2004 to December 2014, 337 adults with pmVSDs underwent transcatheter closure in Xijing Hospital or the General Hospital of Shenyang Military Area Command. Our study included 124 male and 213 female adult patients ranging from 18 to 73 years of age. All patients underwent clinical examinations, chest roentgenography, electrocardiography (ECG), and transthoracic echocardiography (TTE) before intervention.
According to the Chinese consensus and other references from our previous studies, the inclusion criteria for percutaneous closure included (1) a moderate or large left-to-right shunt (Qp/Qs >1.5) causing left heart volume overload that resulted in hemodynamic changes, congestive heart failure, decreased exercise capacity, atrial dysrhythmia, or recurrent bacterial endocarditis; (2) a VSD diameter ≤15 mm as measured through TTE; (3) a distance from the aortic valve to the upper rim of the VSD of ≥1 mm as measured through TTE; (4) a defect positioned from 9 to 11 o’clock across the short-axis parasternal view as determined through TTE; (5) a pulmonary pressure of <70 mm Hg as estimated through TTE; (6) mild aortic valve prolapse and no pathologic aortic regurgitation; (7) no active infective endocarditis; (8) no primary tricuspid chordae tendineae located around the rim of the pmVSD; and (9) no postoperative residual defects or posttraumatic VSD. Patients who were aged 50 yearsor older underwent routine coronary angiography before VSD closure. Before intervention, each patient provided informed written consent. The ethics committees of Xijing Hospital and the General Hospital of Shenyang Military Area Command approved this study, which was registered with ClinicalTrials.gov (number NCT00890799 ) and was conducted in accordance with all relevant Chinese laws.
Two types of occluder devices were used in this study ( Figure 1 ): a double-disk symmetrical concentric pmVSD occluder (S-pmVSO) and a double-disk asymmetrical S-pmVSO (AS-pmVSO; Starway Medical Technology, Inc., Beijing, China). These devices are self-expanding, double-disk devices composed of 0.005-inch nitinol wire mesh with fabric inside. The diameters of the right and left disks were uniformly 4 mm larger than the waist of the S-pmVSO. The diameters of the right and left disks were 4 mm and 8 mm larger than the waist of the AS-pmVSO, respectively. The waist length of the S-pmVSO was 3 mm, and the waist length of the AS-pmVSO was 2 mm. These devices are available in lengths ranging from 4 to 16 mm. Modified double-disk pmVSD occluders were approved in 2005 by the State Food and Drug Administration, People’s Republic of China; they received the Conformité Européenne mark in 2008.
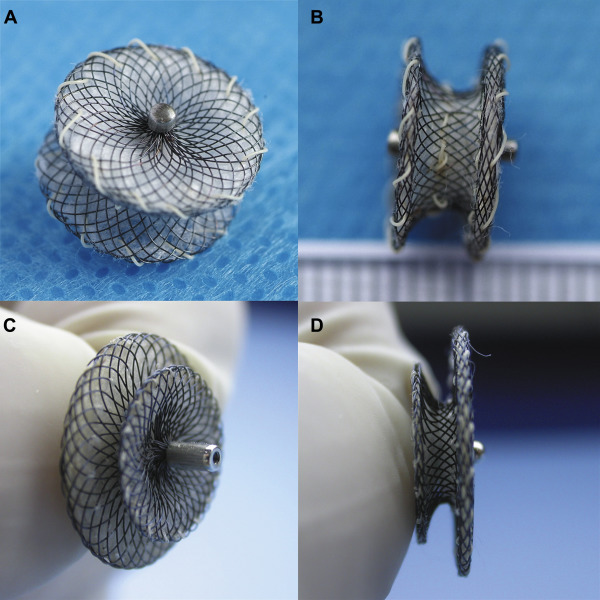
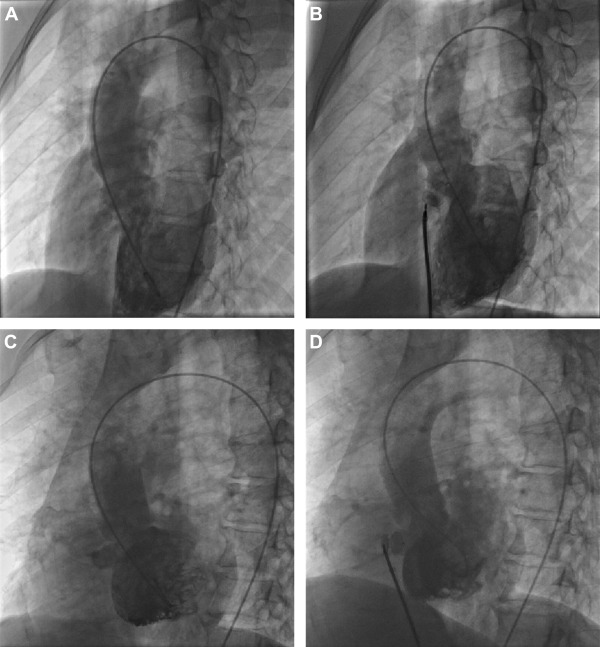
The transcatheter intervention procedure was described in our previous reports, and the ventriculography results are shown in Figure 2 .
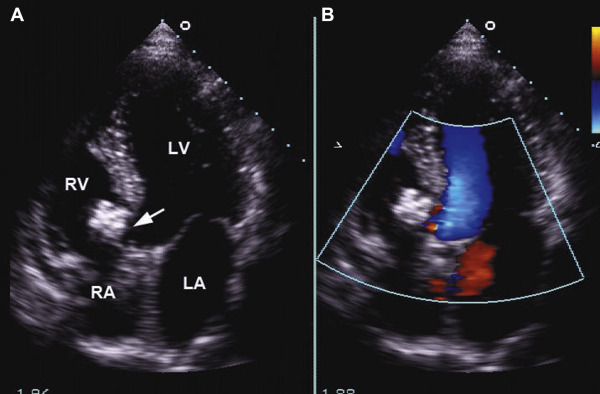
Demographic data, including end-diastolic volume (EDV) and left ventricular end-diastolic dimension (LVEDD) both before the intervention and during the follow-up period, and additional baseline characteristics, were collected from all patients. After the procedure, an ECG was performed on each patient daily until discharge. All patients underwent 24-hour continuous electrocardiographic monitoring and TTE on the third day after the procedure ( Figure 3 ). Follow-up evaluations included clinical examinations, ECG, and TTE at 1, 3, 6, and 12 months and annually thereafter. Adverse events were recorded and classified into categories of major and minor adverse events. Major adverse events included, but were not limited to, death, infective endocarditis, cerebrovascular accidents, complete atrioventricular block (cAVB) requiring either pacemaker implantation or surgical treatment, thromboembolism, and new-onset valvular regurgitation requiring surgical repair. Minor adverse events included, but were not limited to, cardiac arrhythmias requiring medication, new or exacerbated valvular regurgitation <grade 2, mild residual shunts, groin hematoma, hemolysis requiring medication, fever, and rash.
All continuous variables are expressed as either means ± SDs or medians with ranges; discrete variables are presented as either frequencies or percentages. Statistical analysis was performed using SPSS 16.0 for Windows (SPSS, Inc., Chicago, Illinois). Analysis of continuous variables was performed using the Student unpaired t test and 1- and 2-way analysis of variances; analysis of categorical variables was performed using the chi-square test and Fisher exact test. A probability of <0.05 was considered statistically significant. Freedom from major or minor adverse events was assessed using the Kaplan–Meier product-limit method.
Results
A total of 337 patients were enrolled in this study. The S-pmVSO was selected for 302 adults, and the AS-pmVSO was selected for 35 adults. Several basic characteristics were evaluated ( Table 1 ). The median follow-up time was 71 months and ranged from 6 to 137 months. Patients without hemodynamic changes were enrolled primarily because they had significant symptoms, such as exercise intolerance. Four patients had a previous history of infective endocarditis and were free from fever for at least 6 months before intervention. Of the 21 patients who underwent coronary angiography, all had normal results except a 67-year-old man who showed 85% stenosis of the proximal segment of the anterior descending coronary artery and 90% stenosis of the middle segment of the left circumflex coronary artery.
| Variable | Result |
|---|---|
| Male gender | 125 (37.1%) |
| Age (years) | 29.2±10.7 (18–73) |
| Indications | |
| Symptoms (decreased exercise capacity) | 327 (97.0%) |
| Hemodynamic changes (cardiomegaly on chest X-ray, left atrium or left ventricle enlargement verified by echocardiography) | 235 (69.7%) |
| Previous infective endocarditis | 4 (1.2%) |
| Heart function (New York heart association, NYHA grade) | |
| I | 10 |
| II | 253 |
| III | 74 |
| Echocardiography data | |
| Ejection fraction score (%) (range) | 61.45 (44–76) |
| Defect size (mm) (range) | |
| Left side | 10.9±4.2(4–23) |
| Right side | 4.1±1.5(1-8.3) |
| End diastolic volume (ml) (range) | 96.6±23.2 (50–179) |
| Left atrial dimension (mm) (range) | 41.8±7.1 (24–66) |
| Left ventricular end-diastolic dimension (mm) (range) | 50.3±4.7(40–63) |
| Membranous aneurysm | 117 (34.7%) |
| Multioutlet | 44 (13.1%) |
Procedural data are presented in Table 2 . The mean length of the occluders was 10.3 ± 3.4 mm. Deployment of the device was successful in all patients. The type of device used was changed in 3 cases after the initial procedure. Moderate residual shunts remained in 2 patients with multioutlet membranous aneurysms after the placement of an S-pmVSO, but these decreased to mild residual shunts after insertion of an AS-pmVSO. One patient with an AS-pmVSO developed aortic regurgitation, and the subsequent use of an S-pmVSO was successful in this patient. The overall immediate complete closure rate was 63.2% (64.6% in the S-pmVSO group and 51.4% in the AS-pmVSO group; p = 0.141).
| Variable | Result |
|---|---|
| Invasive data | |
| Pulmonary-to-systemic flow ratio | 2.0±0.4 |
| Right atrium mean pressure (mm Hg) | 6.3±1.6 |
| Right ventricle end-diastolic pressure (mm Hg) | 10.9±1.9 |
| Pulmonary artery systolic pressure (mm Hg) | 24.6±3.1 |
| Pulmonary artery diastolic pressure (mm Hg) | 12.2±1.4 |
| Pulmonary artery mean pressure (mm Hg) | 15.7±2.1 |
| Occluder size (mm) | 10.3±3.4 |
| Immediate complete closure rate | 213/337 (63.2%) |
| Fluoroscopic time (min) | 11.2±4.9 |
| Procedure time (min) | 44.0±20.2 |
| Median hospital stay (days) | 6.5±2.6 |
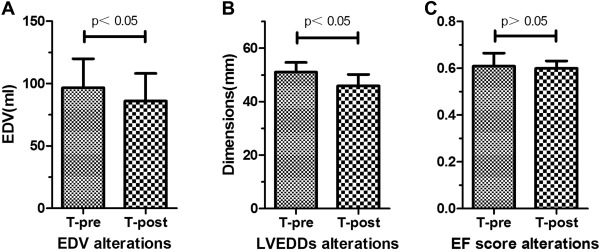
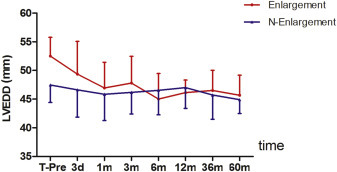
All patients were followed up for at least 6 months: Of these, 314 patients were followed up for >12 months; 219 patients were followed up for >36 months; and 179 patients were followed up for >60 months. The mean LV EDV decreased from 96.6 ± 23.2 to 86.0 ± 22.0 ml (p < 0.05), and the mean LVEDD decreased from 51.0 ± 0.20 to 45.9 ± 0.95 ml (p < 0.05) at the 6-month follow-up visit compared to before intervention. There were no significant changes in ejection fraction after pmVSD closure at the 6-month follow-up visit compared to before intervention (p = 0.645; Figure 4 ).
In patients who were diagnosed with left ventricle enlargement (n = 235) through TTE before their procedures, the mean LVEDD decreased to a normal size during the follow-up period (F = 15.70, p <0.05; Figure 5 ). In patients without left ventricle enlargement (n = 102), LVEDD was not significantly different from that of control subjects. There was no significant difference in LVEDD between the groups of patients with and without left ventricle enlargement after pmVSD closure (F = 1.909, p = 0.168).
The major and minor adverse events are summarized in Table 3 . A total of 2 major adverse events were noted. cAVB was observed in a 35-year-old man 3 days after the placement of a 5-mm S-pmVSO; the patient underwent surgical removal of the device 14 days after the intervention. A 54-year-old woman experienced gradually worsening tricuspid valvular regurgitation and underwent surgical repair 24 months after the intervention.
| Complications | Number |
|---|---|
| Major adverse events | |
| Complete atrioventricular block requiring device surgical removal | 1 (0.3%) |
| New-onset valvular regurgitation requiring surgical repair | 1 (0.3%) |
| Minor adverse events | |
| Mild residual shunt | 11 (3.3%) |
| Arrhythmia rate | 58 (17.2%) |
| Conduction block rate | 41 (12.2%) |
| Left bundle-branch block | 13 (3.9%) |
| Right bundle-branch block | 26 (7.7%) |
| II-atrioventricular block | 2 (0.6%) |
| Ectopic arrhythmia rate | 17 (5.0%) |
| Accelerated idiojunctional rhythm | 15 (4.5%) |
| Accelerated idioventricular rhythm | 2 (0.6%) |
| Tricuspid regurgitation (trivial to small) | 11 (3.3%) |
| Other (e.g., fever >38.5°C, transient headache, rash) | 4 (1.2%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


