Multislice computed tomography (MSCT) is commonly acquired before radiofrequency catheter ablation (RFCA) for atrial fibrillation (AF) to plan and guide the procedure. MSCT allows accurate measurement of the left atrial (LA) and pulmonary vein (PV) dimensions and classification of the PV anatomy. The aim of the present study was to investigate the effect of LA dimensions, PV dimensions, and PV anatomy on the outcome of circumferential RFCA for AF. A total of 100 consecutive patients undergoing RFCA for AF (paroxysmal 72%, persistent 28%) were studied. The LA dimensions, PV dimensions, and PV anatomy were evaluated three dimensionally using MSCT. The PV anatomy was classified as normal or atypical according to the absence/presence of a common trunk or additional veins. After a mean follow-up of 11.6 ± 2.8 months, 65 patients (65%) maintained sinus rhythm. The enlargement of the left atrium in the anteroposterior direction on MSCT was related to a greater risk of AF recurrence. No relation was found between the PV dimensions and the outcome of RFCA. In addition, normal right-sided PV anatomy was related to a greater risk of AF recurrence compared to atypical right-sided PV anatomy. Multivariate analysis showed that an anteroposterior LA diameter on MSCT (odds ratio 1.083, p = 0.027) and normal right-sided PV anatomy (odds ratio 6.711, p = 0.006) were independent predictors of AF recurrence after RFCA. In conclusion, enlargement of the anteroposterior LA diameter and the presence of normal anatomy of the right PVs are independent risk factors for AF recurrence. No relation was found between the PV dimensions and outcome of RFCA.
Multislice computed tomography (MSCT) is now commonly acquired before radiofrequency catheter ablation (RFCA) for atrial fibrillation (AF). MSCT provides important information about the left atrial (LA) and pulmonary vein (PV) anatomy that can be used to plan and guide the RFCA procedure. Moreover, MSCT allows accurate 3-dimensional assessment of LA and PV dimensions. It has been demonstrated that the LA dimensions and PV dimensions are enlarged in patients with AF. However, although the LA size is a well-known risk factor for AF recurrence after RFCA, little is known about the prognostic importance of PV dilation. Similarly, the effect of PV anatomy on the outcome of RFCA has not been extensively studied. Potentially, different anatomic drainage patterns could be accompanied by different tissue characteristics of the surrounding myocardium that might increase the resistance of the PV area to electrical isolation. Moreover, certain anatomic variants could pose a technical difficulty to achieve stable catheter position during ablation, thereby compromising effective lesion formation. The present study describes the effect of LA dimensions, PV dimensions, and PV anatomy assessed by MSCT on the outcome of circumferential RFCA for AF.
Methods
The patient population included 100 consecutive patients with symptomatic drug-refractory AF who had undergone circumferential RFCA. After RFCA, all patients were evaluated on a regular basis at the outpatient clinic during a 12-month follow-up period. Routine electrocardiograms were recorded at each visit and 24-hour Holter registrations were scheduled at 3, 6, and 12 months after ablation. All patients were encouraged to immediately undergo electrocardiography when experiencing palpitations. All medications were continued for ≥3 months. Afterward, antiarrhythmic drugs were discontinued at the discretion of the physician. After a postablation blanking period of 3 months, the recurrence of AF was defined as any recording of AF on an electrocardiogram or an episode >30 seconds on 24-hour Holter monitoring. After 12 months of follow-up, the study population was divided into 2 groups: the patients with maintenance of sinus rhythm (nonrecurrence group) and patients who had experienced recurrence of AF (recurrence group).
Before RFCA, all patients underwent MSCT to guide the procedure. MSCT was performed using a 64-slice Toshiba Aquilion 64 system (Toshiba Medical Systems, Otawara, Japan). A bolus of 70 ml of nonionic contrast material (Iomeron 400, Bracco, Milan, Italy) was infused through the antecubital vein at a rate of 5 ml/s, followed by 50-ml saline solution flush. Automatic detection of the contrast bolus in the descending aorta was used to time the start of the scan. Craniocaudal scanning was performed during breath-hold, without electrocardiographic gating. The collimation was 64 × 0.5 mm, rotation time 400 ms, and the tube voltage 100 kV at 250 mA. After acquisition, the raw MSCT data were exported, postprocessed, and analyzed on a dedicated workstation (Vitrea 2, Vital Images, Minnetonka, Minnesota).
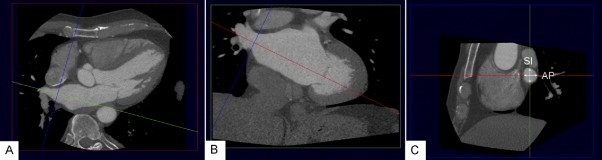
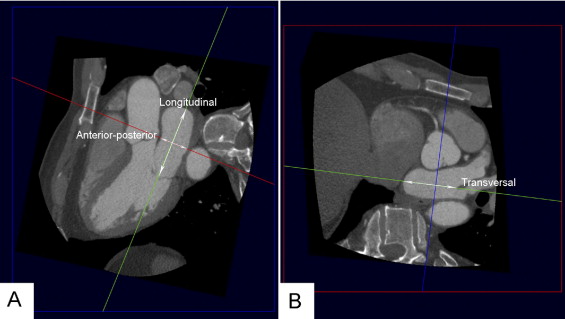
The PV ostium dimensions were evaluated using a 2-dimensional viewing mode. To allow accurate assessment, multiplanar reformatting was used to place 2 orthogonal planes parallel to the course of the vein ( Figure 1 ). The third orthogonal plane, oriented perpendicular to the course of the vein, was then used to measure the diameter of the PV ostium in the anteroposterior and superoinferior direction ( Figure 1 ). The ratio between the largest and smallest diameter was calculated to obtain information on the oval shape of the ostium. Similarly, multiplanar reformatting was used to assess the LA dimensions in 3 orthogonal directions: anteroposterior, longitudinal, and transversal ( Figure 2 ). The LA volume was calculated using the biplane dimension-length formula: LA volume = 4/3π × (anteroposterior diameter/2) × (longitudinal diameter/2) × (transversal diameter/2).
The PV anatomy was analyzed from an external view using a 3-dimensional volume-rendered reconstruction. The PV classification was determined by the presence or absence of a common trunk and/or additional veins ( Figure 3 ). A common trunk was defined when the superior and inferior PV joined >5 mm before entering the left atrium. An additional vein was defined as a supranumerary vein directly entering the left atrium.
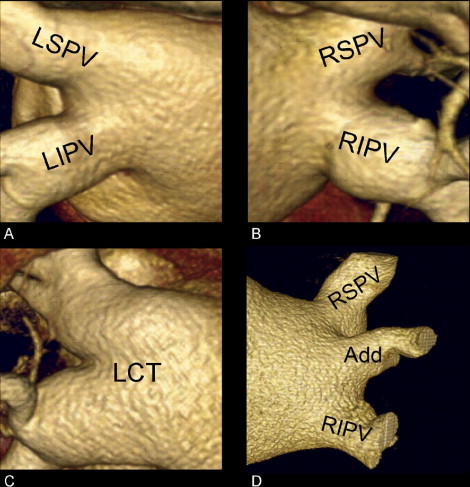
To evaluate the effect of the PV anatomy on the outcome of RFCA for AF, the left- and right-sided anatomy was additionally classified as normal or atypical. Atypical anatomy was defined as the presence of a common trunk or an additional PV.
The RFCA procedure was aimed at creating 2 circumferential lesions around the left- and right-side ipsilateral PVs approximately 1 cm outside the ostia. All patients had received intravenous heparin to maintain an activated clotting time of 300 to 400 seconds. Intracardiac echocardiography was used to guide the trans-septal puncture. To guide the ablation, a nonfluoroscopic electroanatomic mapping system with multislice computed tomographic integration was used (CARTO XP with Cartomerge, Biosense Webster, Diamond Bar, California). Contact mapping and ablation were performed using a 4-mm quadripolar open-loop irrigated mapping/ablation catheter (7Fr, Navistar, Biosense Webster). A 6Fr quadripolar diagnostic catheter placed inside the right atrium served as a temporal reference. A radiofrequency current was applied at 30 to 35 W, with a maximum temperature of 45°C and an irrigation flow of 20 ml/min until a bipolar voltage of <0.1 mV was achieved, with a maximum of 60 seconds per point. The end point of the procedure was PV isolation, as confirmed by recording an entrance block during sinus rhythm or pacing from inside the coronary sinus.
Statistical analyses were performed using the Statistical Package for Social Sciences, version 16.0 (SPSS, Chicago, Illinois). A p value of <0.05 was considered statistically significant. The data are presented as the mean ± SD or as numbers (percentages). Statistical comparisons for continuous variables were performed using the 2-tailed Student t test, paired or unpaired, as appropriate. Statistical comparisons for categorical variables were performed using the chi-square test. Univariate and multivariate logistic regression analyses were performed to study the effect of the clinical characteristics, LA dimensions, and PV dimensions and anatomy on the incidence of AF recurrence after RFCA. The variables with a p value of <0.05 on univariate analyses were included in the multivariate analysis. Multivariate analysis was performed using an “enter” method.
Results
A total of 100 consecutive patients were included (77 men, mean age 56 ± 9 years) from an ongoing clinical registry. The AF was paroxysmal in 72 patients and persistent in 28, according to the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines definitions. None of the patients had previously undergone RFCA for AF. The median duration of AF was 48 months (interquartile range 24 to 84), and the mean number of antiarrhythmic drugs used was 3.1 ± 1.3 per patient. The mean anteroposterior diameter of the left atrium was 43 ± 6 mm, and the mean left ventricular ejection fraction was 58 ± 8% on the transthoracic echocardiogram ( Table 1 ). The procedural end point of PV isolation was achieved in all patients. In 11 patients a repeat procedure was performed owing to early recurrence of AF.
| Variable | Total (n = 100) |
|---|---|
| Age (years) | 56 ± 9 |
| Men | 77 |
| Women | 23 |
| Body surface area (m 2 ) | 2.1 ± 0.2 |
| Atrial fibrillation type (paroxysmal/persistent) | 72/28 |
| Atrial fibrillation duration (months) | 64 ± 60 |
| Antiarrhythmic drugs used per patient | 3.1 ± 1.3 |
| Hypertension | 53 (53%) |
| Coronary artery disease | 8 (8%) |
| Diabetes mellitus | 8 (8%) |
| Echocardiography | |
| Anteroposterior left atrial diameter (mm) | 43 ± 6 |
| Left ventricular ejection fraction (%) | 58 ± 8 |
After a mean follow-up of 11.6 ± 2.8 months, 65 patients (65%) had maintained sinus rhythm (nonrecurrence group), and 35 patients (35%) had had AF recurrence (recurrence group). Antiarrhythmic drug treatment had been discontinued in 16 patients (25%) who had maintained sinus rhythm and in 12 patients (34%) with AF recurrence (p = 0.30). In the recurrence group, a greater prevalence of persistent AF was found than in the nonrecurrence group (14 [22%] vs 14 [40%], p = 0.049).
The LA dimensions were measured in 3 orthogonal directions on the MSCT scans: anteroposterior, longitudinal, and transversal ( Figure 2 ). The mean anteroposterior diameter was 41 ± 7 mm, the mean longitudinal diameter was 65 ± 8 mm, and the mean transversal diameter was 59 ± 7 mm. The anteroposterior LA diameter was significantly larger in the recurrence group than in the nonrecurrence group (43 ± 6 mm vs 39 ± 7 mm, respectively, p = 0.02). No differences were found between the recurrence group and nonrecurrence group in the longitudinal (64 ± 7 mm vs 65 ± 9 mm, p = 0.45) and transversal (59 ± 7 mm vs 60 ± 7 mm, p = 0.57) LA diameter. The mean LA volume was 82 ± 23 cm 3 . The LA volume was larger in the recurrence group than in the nonrecurrence group (88 ± 26 cm 3 vs 78 ± 21 cm 3 , p = 0.04).
The PV ostial dimensions were assessed in the anteroposterior and superoinferior directions on the MSCT scans ( Table 2 ). The PV dimensions were larger in the superoinferior direction than in anteroposterior direction (21.1 ± 2.3 mm vs 16.3 ± 2.5 mm, p <0.001). Overall, the right-sides PVs had a larger diameter than the left-sided PVs (20.0 ± 2.4 mm vs 16.6 ± 1.6 mm, p <0.001). Similarly, superior PVs had a larger diameter than did the inferior PVs (19.6 ± 3.8 mm vs 17.5 ± 3.1 mm, p <0.001). With regard to the shape of the PV ostium, left-sided PVs had a more pronounced oval shape than the right-sided PV ostia, indicated by a lower ratio between the largest and smallest PV diameter (ratio 0.65 ± 0.09 vs 0.84 ± 0.09, p <0.001).
| Variable | Anteroposterior Diameter (mm) | Superoposterior Diameter (mm) | Mean Diameter (mm) | Ratio |
|---|---|---|---|---|
| Right superior pulmonary vein | 18.8 ± 4.2 | 23.3 ± 4.5 | 21.0 ± 4.0 | 0.80 ± 0.11 |
| Right inferior pulmonary vein | 17.7 ± 2.9 | 20.3 ± 3.2 | 19.0 ± 2.8 | 0.87 ± 0.10 |
| Left superior pulmonary vein | 14.3 ± 2.6 | 21.1 ± 3.6 | 17.7 ± 2.4 | 0.67 ± 0.13 |
| Left inferior pulmonary vein | 12.1 ± 2.7 | 18.8 ± 2.5 | 15.4 ± 2.0 | 0.65 ± 0.15 |
| Additional pulmonary vein | 8.9 ± 3.1 | 10.1 ± 2.4 | 9.5 ± 2.5 | 0.82 ± 0.11 |
| Left common trunk | 19.5 ± 4.5 | 33.5 ± 4.7 | 26.5 ± 3.4 | 0.59 ± 0.15 |
| All right pulmonary veins | 18.2 ± 2.5 | 21.8 ± 2.8 | 20.0 ± 2.4 | 0.83 ± 0.08 |
| All left pulmonary veins | 13.2 ± 2.5 | 19.9 ± 2.0 | 16.6 ± 1.6 | 0.66 ± 0.11 |
| All pulmonary veins | 16.3 ± 2.5 | 21.1 ± 2.3 | 18.7 ± 2.2 | 0.77 ± 0.09 |
The PV dimensions were not related to the recurrence of AF during follow-up. No differences were found in the mean PV diameter between the recurrence and nonrecurrence group (18.8 ± 2.2 mm vs 18.6 ± 2.2 mm, p = 0.74). In addition, a similar oval shape of the PV ostia was found in the both groups (nonrecurrence group vs recurrence group, ratio 0.77 ± 0.09 vs 0.77 ± 0.09, p = 0.73).
The PV anatomy was assessed according to the presence/absence of a common trunk and/or additional PV. A total of 174 left-sided PVs and 226 right-sided PVs were identified using MSCT. Separate ostia of the left superior PV and left inferior PV were present in 74 patients (74%) and a common trunk of the left PVs was present in the remaining 26 patients (26%). In addition, separate ostia of the right superior PV and right inferior PV were observed in 78 patients (78%), an additional right-sided PV in 18 patients (18%), and 2 additional right-sided veins in 4 patients (4%). Atypical anatomy of the left-sided PVs was present in 26 patients (26%) and atypical anatomy of the right-sided PVs was observed in 22 patients (22%).
The normal anatomy of the right-sided PVs was associated with a significantly greater risk of AF recurrence compared to atypical anatomy of the right-sided PVs (unadjusted odds ratio [OR] 7.353, p = 0.010). In contrast, the presence of normal or atypical anatomy of the left-sided PVs had no significant effect on the outcome of RFCA (unadjusted OR 2.145, p = 0.14).
Univariate logistic regression analyses were performed to study the effect of LA dimensions, PV dimensions, and PV anatomy, as well as clinical risk factors (e.g., AF type, hypertension) on the outcome of RFCA for AF ( Table 3 ). A large anteroposterior LA diameter (unadjusted OR 1.082, p = 0.021) and a large LA volume (unadjusted OR 1.019, p = 0.045) were related to a greater risk of recurrent AF after RFCA. Similarly, patients with persistent AF had a greater risk of AF recurrence (unadjusted OR 2.429, p = 0.049). The presence of normal right-sided PV anatomy was related to a greater risk of AF recurrence (unadjusted OR 7.353, p = 0.010). Subsequent multivariate analyses demonstrated that anteroposterior LA diameter (OR 1.083, p = 0.027), persistent AF (OR 3.004, p = 0.035), and normal right-sided PV anatomy (OR 6.711, p = 0.006) were independent predictors of AF recurrence ( Table 4 ).



