Mitral regurgitation (MR) is common with coronary artery disease as altered myocardial substrate can affect valve performance. Single-photon emission computed tomography myocardial perfusion imaging (MPI) enables assessment of myocardial perfusion alterations. This study examined perfusion pattern in relation to MR. A total of 2,377 consecutive patients with known or suspected coronary artery disease underwent stress MPI and echocardiography within 1.6 ± 2.3 days. MR was present on echocardiography in 34% of patients, among whom 13% had advanced (moderate or more) MR. MR prevalence was higher in patients with abnormal MPI (44% vs 29%, p <0.001), corresponding to increased global ischemia (p <0.001). Regional perfusion varied in left ventricular segments adjacent to each papillary muscle: adjacent to the anterolateral papillary muscle, magnitude of baseline and stress-induced anterior/anterolateral perfusion abnormalities was greater in patients with MR (both p <0.001). Adjacent to the posteromedial papillary muscle, baseline inferior/inferolateral perfusion abnormalities were greater with MR (p <0.001), whereas stress inducibility was similar (p = 0.39). In multivariate analysis, stress-induced anterior/anterolateral and rest inferior/inferolateral perfusion abnormalities were independently associated with MR (both p <0.05) even after controlling for perfusion in reference segments not adjacent to the papillary muscles. MR severity increased in relation to magnitude of perfusion abnormalities in each territory adjacent to the papillary muscles, as evidenced by greater prevalence of advanced MR in patients with at least moderate anterior/anterolateral stress perfusion abnormalities (10.7% vs 3.6%), with similar results when MR was stratified based on rest inferior/inferolateral perfusion (10.4% vs 3.0%, both p <0.001). In conclusion, findings demonstrate that myocardial perfusion pattern in left ventricular segments adjacent to the papillary muscles influences presence and severity of MR.
This study examined myocardial perfusion pattern in relation to mitral regurgitation (MR) among a consecutive cohort of 2,377 patients with known or suspected coronary artery disease (CAD) who underwent stress myocardial perfusion imaging (MPI) and echocardiography (echo). The goal was to test the interaction between altered myocardial perfusion and both presence and severity of MR.
Methods
The study population consisted of consecutive patients who underwent single-photon emission computed tomography (SPECT) MPI and transthoracic echo within a 1-week interval at Weill Cornell Medical College. Imaging was performed between December 2010 and December 2013. To test the impact of myocardial perfusion pattern on MR, patients with primary mitral valve disorders (mitral valve prolapse or rheumatic disease) or previous mitral valve surgery (prosthesis or annuloplasty) were excluded. This study was conducted with approval of the Weill Cornell Medical College Institutional Review Board.
MPI was performed in accordance with a previously described protocol. In brief, thallium-201 (Tl-201; ∼3 mCi) or technetium-99m (Tc-99m; ∼10 mCi) sestamibi was injected intravenously; baseline (i.e., rest) perfusion images were acquired approximately 10 minutes after Tl-201 injection and 60 minutes after Tc-99m sestamibi injection. After baseline imaging, patients capable of exercise underwent treadmill testing using a Bruce protocol: Tc-99m (∼30 mCi) sestamibi was intravenously administered at peak stress after achievement of target heart rate response to exercise (≥85% age-predicted maximum heart rate). Serial 12-lead electrocardiograms (ECGs) were obtained at baseline and at each stage of the exercise treadmill protocol. In patients unable to exercise or to achieve adequate exercise heart rate response, pharmacologic protocols were employed using either intravenous adenosine-based agents or dobutamine. Poststress images were acquired approximately 30 minutes after exercise and 1 to 2 hours after pharmacologic stress.
SPECT imaging was performed using a dual headed scintillation camera system with a low-energy high-resolution collimator. Images were acquired using a 180° arc of rotation along a circular orbit encompassing a total of 64 projections. For Tl-201 imaging, 2 photopeaks of 70 and 167 keV were used. For Tc-99m imaging, a photopeak of 140 keV was used. Stress images were ECG gated for assessment of contractile function; left ventricular (LV) ejection fraction was quantitatively measured using Cedars-Sinai AutoQuant (Philips Healthcare, Andover, MA).
Echoes were performed by experienced sonographers using commercially available equipment (e.g., Vivid 7 [General Electric, Fairfield, CT], iE33 [Philips Healthcare, Andover, MA]). Images were acquired in parasternal as well as apical 2-, 3-, and 4-chamber orientations. LV ejection fraction and chamber size were quantified using linear dimensions in parasternal views. Color and pulsed wave Doppler were used to evaluate the presence and severity of MR.
MPI was interpreted by the American Heart Association/American College of Cardiology level III trained readers using a 17-segment model. Perfusion defect severity on a per-segment basis was graded using a 5-point scoring system (0 = normal perfusion, 1 = equivocal or mildly reduced, 2 = moderately reduced, 3 = severely reduced, and 4 = absence of detectable radioisotope). Summed stress and rest scores were calculated by adding per-segment defect severity for all segments. Inducible defect severity (summed difference score) was assessed as the difference between rest and stress images.
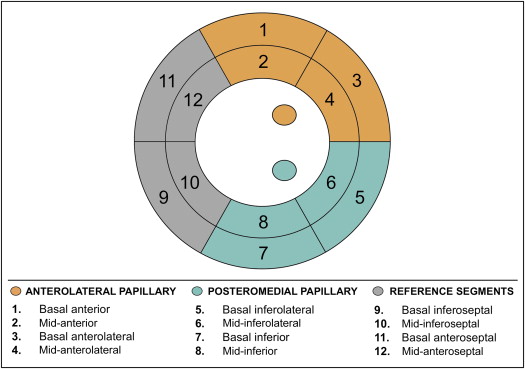
To test the relation between mitral apparatus ischemia and MR, regional perfusion was assessed within myocardial segments subtending the anterolateral and posteromedial papillary muscles ( Figure 1 ). For the anterolateral papillary muscle, LV perfusion was assessed within the basal to mid-anterior and basal to mid-anterolateral segments. For the posteromedial papillary muscle, LV perfusion was assessed within the basal to mid-inferior and basal to mid-inferolateral segments. As a reference for LV regions not adjacent to the papillary muscles, perfusion was assessed within the basal to mid-anteroseptal and basal to mid-inferoseptal segments. Perfusion to each region was additionally graded based on the maximal perfusion score within the region: regions with completely normal perfusion were scored as normal, regions with a maximum perfusion score of 1 in the constituent segments were scored as mildly abnormal, and regions with a maximum perfusion score of ≥2 in the constituent segments were scored as at least moderately abnormal.
Echoes were interpreted by experienced (American Heart Association/American College of Cardiology level III) readers in a high-volume laboratory, for which methods of measurement of chamber volumes and MR have been previously reported. MR severity was graded using a 5-point scale, as primarily determined based on the distance reached from the mitral orifice by the regurgitant jet (mild [1+]; ≤1.5 cm | moderate [2+]; 1.5 to 3.0 cm | moderately severe [3+]; 3.0 to 4.5 cm | severe [4+]; ≥4.5 cm). Additional criteria used to confirm MR severity included jet area and vena contracta as well as mitral and pulmonary vein flow pattern. Pulmonary artery systolic pressure was calculated from tricuspid regurgitant velocity and inferior vena cava caliber.
Comparisons between groups with and without MR were made using Student’s t test for continuous variables (expressed as mean ± SD). Indices were tested for normality of distribution; non-normally distributed data (i.e., perfusion scores) were compared after logarithmic transformation for which results are expressed as the antilog of the mean and 95% confidence intervals (CIs). Categorical variables were compared using chi-square or, when fewer than 5 expected outcomes per cell, Fisher’s exact test. Multivariable logistic regression analysis was performed to evaluate associations between MR and SPECT perfusion pattern. Binary logistic regression analysis was used to examine the association between MR, clinical variables, and imaging parameters. Two-sided p <0.05 was considered indicative of statistical significance. Calculations were performed using SPSS, version 20 (IBM, Armonk, NY).
Results
The study population comprised 2,377 consecutive patients without primary mitral valve disease who underwent MPI and echo within a 1-week (1.6 ± 2.3 days) interval. A total of 80 otherwise eligible patients were excluded based on echo-evidenced primary mitral valve disease (57% prolapse and 13% rheumatic) or previous mitral valve surgery (30% prosthesis or annuloplasty).
MR was present in 1/3 (34%) of the study population (87% mild, 8% moderate, 3% moderate-severe, and 2% severe). Table 1 details population clinical and imaging characteristics, stratified based on the presence or absence of MR. As shown, MR was strongly associated with clinically established CAD as evidenced by a near twofold increase in prevalence of previous MI or coronary artery bypass grafting (CABG) in MR-affected patients (both p <0.001). Regional LV contractile dysfunction on echo was also more common in patients with MR, who manifested increased prevalence of regional wall motion abnormalities in all coronary vascular territories (all p <0.001). Accordingly, LV ejection fraction was lower in patients with MR, whether measured via baseline echo or poststress SPECT imaging (both p <0.001).
| Parameter | Overall (n = 2377) | Mitral Regurgitation | p | |
|---|---|---|---|---|
| Yes (n = 812) | No (n = 1565) | |||
| Age (years) | 64 ± 13 | 68 ± 13 | 62 ± 13 | <0.001 |
| Men | 1271 (54%) | 418 (52%) | 853 (55%) | 0.16 |
| Hypertension † | 1707 (72%) | 629 (78%) | 1078 (69%) | <0.001 |
| Hypercholesterolemia † | 1414 (60%) | 483 (60%) | 931 (60%) | 0.99 |
| Diabetes mellitus | 759 (32%) | 246 (30%) | 513 (33%) | 0.22 |
| Family history of coronary artery disease | 563 (24%) | 169 (21%) | 394 (25%) | 0.02 |
| Known coronary artery disease | 552 (23%) | 241 (30%) | 311 (20%) | <0.001 |
| Prior myocardial infarction (MI) | 177 (7%) | 82 (10%) | 95 (6%) | <0.001 |
| Prior coronary revascularization | ||||
| Percutaneous coronary intervention (PCI) | 344 (15%) | 136 (17%) | 208 (13%) | 0.02 |
| Coronary artery bypass grafting (CABG) | 176 (7%) | 96 (12%) | 80 (5%) | <0.001 |
| Medications | ||||
| Beta-blocker | 1148 (48%) | 459 (57%) | 689 (44%) | <0.001 |
| ACE inhibitor or angiotensin receptor blocker | 940 (40%) | 364 (45%) | 576 (37%) | <0.001 |
| HMG-CoA reductase inhibitor | 1237 (52%) | 469 (58%) | 768 (49%) | <0.001 |
| Aspirin | 1164 (49%) | 435 (54%) | 729 (47%) | 0.001 |
| Thienopyridine | 289 (12%) | 110 (14%) | 179 (11%) | 0.14 |
| Indication for stress perfusion testing | ||||
| Chest pain | 984 (41%) | 298 (37%) | 686 (44%) | 0.001 |
| Dyspnea | 679 (29%) | 242 (30%) | 437 (28%) | 0.34 |
| SPECT myocardial perfusion imaging | ||||
| Exercise stress | 907 (38%) | 245 (30%) | 662 (42%) | <0.001 |
| Adenosine/regadenoson stress | 1465 (62%) | 564 (70%) | 901 (58%) | <0.001 |
| Dipyridamole stress | 2 (0.1%) | 2 (0.2%) | — | 0.12 |
| Dobutamine stress | 3 (0.1%) | 1 (0.1%) | 2 (0.1%) | 1.00 |
| Poststress ejection fraction (%) | 63 ± 13 | 60 ± 15 | 64 ± 12 | <0.001 |
| Poststress ejection fraction <50% | 270 (11%) | 159 (20%) | 111 (7%) | <0.001 |
| Echocardiography | ||||
| LV ejection fraction (%) | 61 ± 11 | 58 ± 14 | 62 ± 9 | <0.001 |
| LV ejection fraction <50% | 320 (14%) | 167 (21%) | 153 (10%) | <0.001 |
| LV end-diastolic diameter (cm) | 5.0 ± 0.8 | 5.1 ± 0.9 | 5.0 ± 0.8 | <0.001 |
| LV regional wall motion abnormality | 299 (13%) | 157 (19%) | 142 (9%) | <0.001 |
| Anterior wall motion abnormality | 117 (5%) | 71 (9%) | 46 (3%) | <0.001 |
| Lateral wall motion abnormality | 175 (7%) | 104 (13%) | 71 (5%) | <0.001 |
| Inferior wall motion abnormality | 219 (9%) | 133 (16%) | 86 (6%) | <0.001 |
| LV myocardial mass (g/m 2 ) | 92 ± 32 | 100 ± 35 | 87 ± 30 | <0.001 |
| Left atrial diameter (cm) | 3.9 ± 1.4 | 4.1 ± 0.8 | 3.8 ± 1.6 | <0.001 |
| Left atrial volume (ml/m 2 ) | 34 ± 14 | 40 ± 16 | 30 ± 11 | <0.001 |
† Defined based on self-reported history, confirmed via medical record review at time of MPI.
MR was more common in patients with, compared to those without, abnormal myocardial perfusion on SPECT (44% vs 29%, p <0.001); differences were more marked with respect to advanced (moderate or more) MR (8% vs 2%, p <0.001). Among patients who underwent exercise stress (n = 907), ECG stress response was more frequently abnormal in those with MR, as evidenced by higher prevalence of exercise-induced horizontal or downsloping (≥1.0 mm) ST-segment depression (20% vs 14%, p = 0.03).
As shown in Table 2 , patients with MR had increased severity of impaired perfusion on imaging at rest and greater magnitude of stress-induced perfusion abnormalities (both p <0.001). Although ancillary SPECT findings were uncommon, prevalence of stress-induced transient ischemic dilation was similar between patients with and without MR (2.0% vs 1.3%; p = 0.19), whereas increased lung uptake—a marker of elevated LV filling pressure—was more frequent with MR (3.8% vs 0.4%, p = 0.001).
| Mitral Regurgitation | ≥Moderate Mitral Regurgitation | |||||
|---|---|---|---|---|---|---|
| Present (n = 812) | Absent (n = 1565) | p | Present (n = 104) | Absent (n = 2273) | p | |
| Left ventricular global perfusion | ||||||
| Summed stress score | 2.68 (2.47–2.92) | 1.83 (1.74–1.93) | <0.001 | 4.99 (3.85–6.47) | 2.00 (1.92–2.09) | <0.001 |
| Summed rest score | 2.14 (1.99–2.32) | 1.54 (1.48–1.61) | <0.001 | 3.65 (2.82–4.71) | 1.67 (1.60–1.73) | <0.001 |
| Summed difference score | 1.55 (1.46–1.64) | 1.33 (1.29–1.37) | <0.001 | 2.04 (1.70–2.45) | 1.38 (1.34–1.42) | <0.001 |
| Mitral apparatus perfusion | ||||||
| Anterolateral | ||||||
| Summed stress score | 1.35 (1.29–1.41) | 1.18 (1.15–1.21) | <0.001 | 1.61 (1.39–1.87) | 1.22 (1.19–1.24) | <0.001 |
| Summed rest score | 1.14 (1.11–1.17) | 1.07 (1.05–1.08) | <0.001 | 1.25 (1.12–1.40) | 1.08 (1.07–1.10) | 0.01 |
| Summed difference score | 1.23 (1.19–1.27) | 1.12 (1.10–1.14) | <0.001 | 1.41 (1.25–1.58) | 1.14 (1.12–1.16) | 0.001 |
| Posteromedial | ||||||
| Summed stress score | 1.78 (1.68–1.90) | 1.41 (1.36–1.46) | <0.001 | 2.50 (2.05–3.04) | 1.49 (1.45–1.54) | <0.001 |
| Summed rest score | 1.64 (1.55–1.74) | 1.31 (1.26–1.35) | <0.001 | 2.30 (1.90–2.79) | 1.38 (1.34–1.42) | <0.001 |
| Summed difference score | 1.15 (1.11–1.18) | 1.13 (1.11–1.15) | 0.39 | 1.16 (1.07–1.26) | 1.14 (1.12–1.16) | 0.60 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


